摘要/Abstract
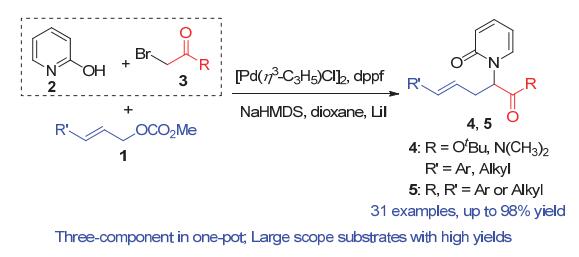
N-酰亚甲基-2-吡啶酮是一类非常重要的结构单元, 广泛存在于天然产物和其它具有生物活性的化合物中. 其合成通常是通过2-羟基吡啶与相应的亲电试剂发生分子间亲核取代反应. 然而, 由于2-羟基吡啶的双亲核特性, 这一方法往往面临着N/O化学选择性难以控制的问题. 报道了一例钯催化三组分烯丙基取代反应, 化学专一性地合成难以构建的大位阻N-酰亚甲基-2-吡啶酮衍生物, 收率最高可达98%, 未见有O-烷基化副产物的生成. 该反应可以在克级规模下进行, 依然取得98%的收率. 本方法所得到的N-酰亚甲基-2-吡啶酮产物经过简单转化, 可方便地制得含吡啶酮结构的非天然氨基酸类化合物. 实验结果显示, 该三组分反应是经过一个串联的亲核取代和烯丙基取代反应而专一性地合成N-酰亚甲基-2-吡啶酮衍生物.
关键词: 烯丙基串联反应, 三组分, 化学专一性, N-酰亚甲基-2-吡啶酮
Functionalized N-carbonylmethylene-2-pyridones are some of the most important structural motifs and exist in many natural products and bioactive compounds. Thus, the efficient construction of such skeletons has attracted much attention. Generally, the synthesis of N-carbonylmethylene-2-pyridones is realized via an intermolecular nucleophilic substitution of 2-hydroxypyridines and appropriate electrophiles. However, the above reactions often suffer from low yields caused by poor O/N chemoselectivities due to the dual nucleophilicity of the 2-hydroxypyridines. As far as the structure is concerned, N-carbonylmethylene-2-pyridones can be divided into three sections: a pyridone, a carbonylmethyl group and a side chain. When the side chain is a H atom, the N-substituted pyridones can be constructed conveniently via a reaction of 2-hydroxypyridines and primary α-bromocarbonyl compounds in high yields with excellent chemoselectivities. However, when the side chain is not a H atom, for example an alkyl group, only limited examples have been reported and only moderate yields of the desired N-substituted pyridine products are obtained by a combination of 2-hydroxypyridines and bulky secondary α-bromocarbonyl compounds, mainly due to the poor O/N chemoselectivities. To achieve a general synthetic pathway for the latter, the following practical strategy was designed. 2-Hydroxypyridines were first treated with primary α-bromocarbonyl compounds to generate the unique N-substituted intermediates in situ, which then reacted with the side chain electrophiles to give only the N-alkylated final products. Thus, a Pd-catalyzed three-component chemospecific allylic substitution cascade has been developed for the synthesis of N-carbonylmethylene-2-pyridone derivatives, with the desired products being obtained in up to 98% yield. No O-alkylated by-product was observed. The results suggested that the N-carbonylmethylene-2-pyridones are constructed via a cascade reaction consisting of a nucleophilic substitution followed by an allylic alkylation. The reaction was performed on a gram scale and the corresponding alkylated product was conveniently converted to a pyridone-containing unnatural amino acid. This methodology allows for the highly chemoselective synthesis of biologically important N-carbonylmethylene-2-pyridone derivatives.
Key words: allylic substitution cascade, three-component, chemospecificity, N-carbonylmethylene-2-pyridone
PDF全文下载地址:
点我下载PDF
