摘要/Abstract
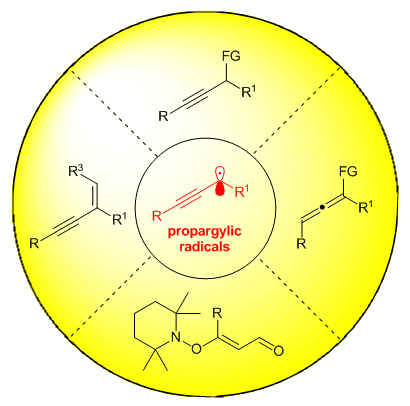
炔烃的合成与转化一直是有机合成化学的一个重要研究内容. 其中, 炔丙位官能化是实现炔烃合成与转化的一个重要途径. 相对于经历阳离子中间体途径的炔丙位官能化反应, 自由基途径的炔丙位官能化反应在最近十年才得以发展, 且与前者也已形成互补之势. 该类炔丙基自由基既能够通过炔丙位的碳杂键断裂生成, 又可通过自由基对1,3-烯炔的加成生成. 同时, 由于炔丙基自由基存在自由基与炔烃的共轭结构, 使得该自由基既能够直接对金属物种加成参与炔丙位的官能化反应, 又能够异构成联烯自由基后对金属物种加成, 继而参与联烯化合物的合成. 此外, 炔丙基自由基还可以被进一步氧化成炔丙基阳离子后参与后续的有机转化. 本综述根据炔丙基自由基所参与的反应类型, 对近年来炔丙位自由基参与的有机反应进行了简要总结.
关键词: 炔丙基自由基, 炔丙位官能化, 炔烃, 联烯, 过渡金属催化
The production and transformation of alkynes occupys an important position in organic synthetic chemistry. Within this realm, propargylic functionalization of alkynes is a feasible way towards this purpose. Especially, the propargylic functionalization via radical pathways has flourished in the last decade, which is believed to be a significant complement to the classic metal-catalyzed propargylation reaction involving cationic intermediates. According to the reaction modes, these advancements will be highlighted by classifying into four types. The first one is the propargylic functionalization reactions involving propargylic radicals. Generally, propargylic radicals can be generated through single electron reduction of alkyne substrates by low-valence metal catalysts or excited state of photocatalysts, then participated in the following cross-coupling reactions to achieve alkyne products. In this part, asymmetric variants have been also well developed. The second one is the preparation of allene compounds through the allenyl radical pathway. For these processes, propargylic radicals can isomerize to allenyl radicals, which can participate in the copper- or nickel-catalyzed coupling reaction to produce significant allene compounds. The third one is the dehydrative alkylation reaction of propargyl alcohols that involve propargylic radical intermediates, too. Such radical intermediates can be further oxidized to propargylic cation intermediates, followed by a deprotonation to form substituted 1,3-enyne compounds. The forth one is the synthesis of vinylic alkoxyamines through a propargylic radical route. Initially, propargyl alcohols can be converted to propargylic radical species by the joint action of copper catalysts and TEMPO. The generated propargylic radical species can be captured by TEMPO to form vinylic alkoxyamines. Finally, an outlook on the radical propargylic functionalizations will be provided at the end of this review.
Key words: propargylic radical, propargylic functionalization, alkyne, allene, transition metal catalysis
PDF全文下载地址:
点我下载PDF
