摘要/Abstract
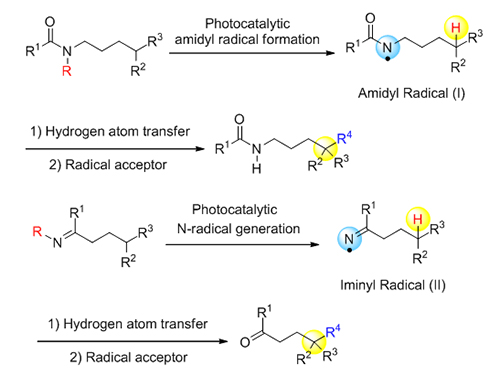
远程sp 3碳氢键官能团化反应近年来引起广泛关注, 可见光催化的氮自由基导向选择性官能化这一技术的出现使得该领域的发展取得了可喜的进展. 该策略以氮自由基介导的Hoffman-L?ffler-Freytag (HLF)反应为基础, 通过在可见光照射下激发态自由基的生成和1,5-攫取氢原子(1,5-HAT)过程, 利用反应过程中形成的自由基中间体, 实现对远程sp 3碳-氢键的修饰. 本综述就可见光催化的氮自由基导向远程碳氢官能团化反应近年来取得的进展进行简要总结.
关键词: 可见光, 自由基, 远程官能团化
The selective functionalization of unactivated sp 3 carbon-hydrogen (C—H) bond is an attractive strategy in modern organic transformation. The hydrogen atom transfer (HAT) catalysis has recently shown its advances in remotely selective activation of an inert C—H bond with great functional group compatibility, generating new carbon-carbon (C—C) bonds and carbon-heteroatom (C—O, C—N, C—X) bonds. Therefore, the remote sp 3 C—H functionalization has become an intensively investigated research area, drawing extensive attention in synthetic community. Particularly, the 1,5-hydrogen atom abstraction of nitrogen radicals, the key step of the Hoffman-L?ffler-Freytag (HLF) reaction, has been widely applied in the preparation of heterocycles. Comparing to the well-studied area of nucleophilic N-species, N-centered radical based reactions are still underdeveloped. The limited utility is partially due to the required use of hazardous radical initiators, elevated temperatures, or high-energy UV irradiation for the generation of N-radicals. Recently, visible-light photoredox catalysis has been leading efficient accesses to Nitrogen-radicals under mild conditions. The visible-light-induced nitrogen radical formation has also stimulated the development of the remote sp 3 C—H functionalization by photoredox catalysis. The classic HLF reaction requires pre-functionalization at N-center in the substrate to promote the formation of N-radical. Recently, a direct N—H single electron transfer (SET) oxidation was realized by photoredox catalysis in Knowles and Rovis’s group, generating N-radicals efficiently. The processes significantly simplified the preparation of the HLF reaction substrates and broaden the application of this classic reaction. In addition, the visible-light-induced nitrogen radical-directed reaction on modified imines provided possibilities for the remote sp 3 C—H functionalization of ketones, as ketone is the product of imine hydrolysis. Moreover, the application of chiral Lewis acid catalysis combined with visible-light photoredox catalysis enabled the asymmetric alkylation of the unactivated remote sp 3 C—H position, which achieves both regioselective and stereoselective functionalization. In conclusion, this strategy takes advantage of mild generation of N-radicals upon visible-light excitation. Subsequent 1,5-hydrogen atom transfer (1,5-HAT) and intermolecular radical coupling would realize the remote functionalization of unactivated sp 3 C—H bonds. The strategies have been successfully applied in remote C(sp 3)—H amidation, fluorination, chlorination, iodination, alkylation, vinylation, allylation, oxygenation, thioetherification, cyanation and alkynylation. In this review, we focus on visible-light-induced nitrogen radical directed functionalization of remote sp 3 C—H bonds, summarized the methodologies, and briefly reviewed their synthetic applications in pharmaceuticals and natural products.
Key words: visible-light, radical, remote functionalization
PDF全文下载地址:
点我下载PDF
