摘要/Abstract
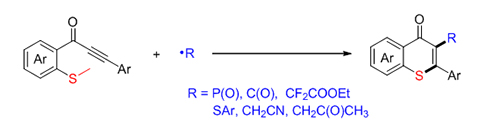
硫代吡喃酮是一种重要的结构骨架, 广泛存在于天然产物、潜在药物及生物活性分子中. 因此, 发展简洁、高效构建硫代吡喃酮的合成方法具有重要意义. 发展了一种自由基促进的硫甲基取代炔酮的加成环化反应来构建硫代吡喃酮环的新方法. 该方法具有底物适用性广, 一系列自由基前体如二苯基膦氧、硫酚、醛等都可以在该反应体系中实现转化. 机理研究表明, 自由基前体对炔酮的选择性加成得到C(sp 2)自由基中间体, 该中间体促进的C(sp 2)—S键构建及C(sp 3)—S键断裂是关键步骤.
关键词: 自由基, 硫代吡喃酮, 环化反应, 自由基串联反应
Thiochromones are prevalent structures in various biological active molecules, natural products and potent drug candidates. However, only few methods for the synthesis of thiochromones were reported, and the traditional methods suffer from harsh conditions such as high temperature, strong acid, etc. Recently, synthesis of thiochromones from alkynones had been independently developed by the group of Larock, Mu?ller and Fu. Compared to traditional substances, alkynones are easy to be prepared and handled. More recently, Wu and co-authors improved this synthetic approach via a palladium-catalyzed carbonylative four-component reaction. Despite these great advances, syntheses of diversely functionalized thiochromones, especially 2-functionalized thiochromones which were not easily prepared via the above approaches, are still in demand and highly desirable. As part of our on-going interest in the synthesis of heterocyclic compounds via radical cascade reactions, herein, we developed a radical-involved annulation of methylthiolatedalkynones with diverse radical precursors to access 2-substituted thiochromones. Various substituents such as F, Br and OMe on aromatic ring were all compatible with the reaction, affording the desired 2-substituted thiochromones in moderate to good yields. The most advantage of this protocol is the compatibility of diverse radical precursors including H-phosphorus oxides, aryl aldehydes, arylthiols, BrCF2COOEt, acetone and acetonitrile. Moreover, a series of control experiments were performed to interpret the reaction pathway as a radical process instead of electrophilic cyclization process. Mechanism studies showed that radical involved C(sp 2)—S bond formation and C(sp 3)—S cleavage are the key steps. A general procedure for the radical annulation of alkynones with acetone and acetonitrile is as followed. To the mixture of alkynones 1 (0.2 mmol), in a schlenk flask was added a solution of tert-butyl peroxybenzoate (TBPB) (0.4 mmol) in acetone or acetonitrile (2 mL) under nitrogen atmosphere. The reaction was stirred at 130 or 120 ℃ for 12 h. Upon completion, the reaction mixture was concentrated under vacuum. The residue was purified by silica gel column chromatography using a petroleum ether/ethyl acetate (V:V, 8:1~5:1) to afford the corresponding products 6.
Key words: radical, thiochromen, cyclization reaction, radical cascade reaction
PDF全文下载地址:
点我下载PDF
