摘要/Abstract
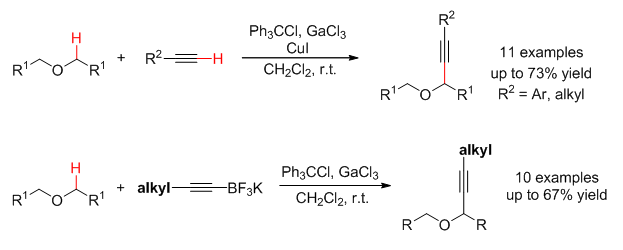
首次报道了饱和开链醚和末端炔的交叉脱氢偶联.在Ph3CCl/GaCl3介导的温和氧化条件下,饱和开链醚与一系列芳基和烷基末端炔在室温可发生交叉脱氢偶联,反应收率高达73%.鉴于烷基乙炔反应效率较低的问题,还报道了Ph3CCl/GaCl3介导的饱和开链醚和一系列烷基取代的炔基硼试剂的氧化偶联,并获得了较好的反应收率.
关键词: 饱和开链醚, 末端炔, 交叉脱氢偶联, 碳正离子
C—C bond forming reactions through cross-dehydrogenative coupling (CDC) of two readily available C—H components under oxidative conditions have emerged as one of the most straightforward and economical approaches for increasing molecular complexity and functional group content with minimal waste generation. CDC reactions involving oxidative functionalization of sp3 C—H bonds of both cyclic and acyclic amines with diverse partners have been extensively explored. In sharp contrast, the CDC of corresponding ether substrates remains relatively underdeveloped. Current approaches predominantly focus on cyclic ethers as well as acyclic benzylic ethers. The CDC reaction of extensively existing unactivated acyclic ethers proved to be much more challenging, which might be ascribed to their inherent low reactivity. On the other hand, the existing protocols for unactivated ethers rely heavily on peroxide-mediated oxidation systems, which typically required high temperature and a large excess of ether substrates as the solvent. Accordingly, coupling partners that can be compatible with such harsh conditions are largely restricted to sp2 or sp3 C—H reagents with relatively low manipulation capability, such as arenes, heteroarenes, and 1,3-dicarbonyl moieties. Alkynes represent common structural motifs spread across the fields of biology, chemistry, material science, and medicine, and act as global handles for diverse functionalities. Therefore, the development of a mild approach for CDC of unactivated acyclic ethers with terminal alkynes is highly desired. In 2014, our group developed a mild Ph3CCl/GaCl3 mediated oxidation system, allowing to achieve the oxidative C—H alkynylation of tetrahydrofuran with organoboranes. Herein, we reported the first CDC of unactivated acyclic ethers with terminal alkynes promoted by Ph3CCl/GaCl3. The reaction proceeded at room temperature in CH2Cl2, thus avoiding the employment of excess ether as the solvent. The typical procedure is as follows:a mixture of unactivated acyclic ether (2.0 mmol), terminal alkyne (0.1 mmol), Ph3CCl (0.1 mmol), and CuI (0.03 mmol) in CH2Cl2 at r.t. was added GaCl3 (0.1 mmol) in a glove box to afford the expected coupling products in moderate to good yields. The Ph3CCl/GaCl3 mediated oxidative C—H alkynylation of unactivated acyclic ethers with alkyl substituted alkynylboranes was further established to overcome the relative low efficiency for the CDC reaction involving alkyl substituted terminal alkynes.
Key words: acyclic ether, unactivated ether, terminal alkyne, cross-dehydrogenative coupling, carbocation
PDF全文下载地址:
点我下载PDF
