摘要/Abstract
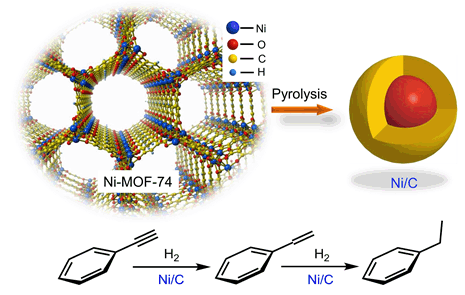
通过水热合成法以硝酸镍和2,5-二羟基对苯二甲酸为原料成功合成Ni-MOF-74.以此为前体,在Ar气氛下热解制备了一系列介孔结构丰富、尺寸均匀的纳米Ni/C核壳催化剂.Ar气氛下通过延长热解时间(400℃,6 h)或提高热解温度(≥500℃)可以得到完全热解产物,粒子尺寸随热解温度的升高而增大.H2-TPR结果表明随着热解温度的提高,镍与表面碳层之间的相互作用增强,材料还原性能降低.TEM和Ar离子溅射XPS给出了Ni/C纳米材料为核壳结构的直接证据.由于表面碳层的化学惰性,隔离作用和电子调控作用,使得部分镍物种能够以单质镍(Ni0)形式稳定存在.其次,表面碳壳的存在减弱了颗粒间的相互作用,有利于催化剂在反应体系中均匀分散.以苯乙炔选择加氢反应为探针,在高压釜式反应器中测试Ni/C的催化性能.研究发现Ni/C在苯乙炔加氢反应中表现出了非常优异的催化活性(0.833 mmol•min-1•gcat.-1)和稳定性.其与几种催化剂的加氢活性由弱到强的顺序为:Ni < NiSix < 负载型Ni2Si < Ni/C < Pd < Pt.
关键词: MOF热解, Ni/C纳米材料, 核壳结构, 多相催化, 苯乙炔加氢
A series of Ni/C core-shell nano catalysts with abundant mesoporous and uniform size were prepared by Ni-MOF-74 pyrolysis. The Ni-MOF-74 was synthesized via hydrothermal method with nickel acetate and 2,5-dihydroxyterephthalic acid (DHTA) as raw materials. The pyrolysis process was carried out in a tube furnace under Argon (Ar) atmosphere with a heating rate of 2℃/min. Completed pyrolytic product Ni/C can be obtained by extending the pyrolysis time (6 h) at 400℃ or increasing the pyrolysis temperature (≥ 500℃) based on the TG result. Moreover, the particle size of Ni/C varied with pyrolysis temperature from 3 nm (500℃) to 17 nm (800℃). The TEM images and Ar ion sputtering XPS indicated a core-shell structure of the pyrolysis product. Nickel species can be stable in the form of nickel (Ni0) due to the electronic properties regulating and confinement effect of the carbon shell. Moreover, the carbon shell greatly weaken the interaction between particles, which is favorable for the dispersion of the catalyst in the reaction system. H2-TPR results show that the interaction between nickel and amorphous carbon increases with the pyrolysis temperature, which is unfavorable to the interaction between Ni species and the reactant. The phenylacetylene (PA) hydrogenation reaction was carried out with 0.2 g catalyst, 10 mL of 1 mol/L ethanolic phenylacetylene solution and 1.0 MPa H2 in a 50 mL high-pressure autoclave under 50℃. Ni/C exhibits excellent catalytic activity and recyclability in phenylacetylene (PA) hydrogenation. In addition, we compared the activity of Ni/C with several reported catalyst system and found their activity increases in the order of Ni, NiSix, supported Ni2Si, Ni/C, Pd and Pt. With an activity of up to 0.833 mmol•min-1•gcat.-1 at 50℃ (Ni/C-400-6, Ni/C-500-2), Ni/C is the most promising transition metal catalyst that can be comparable with noble metal.
Key words: MOFs pyrolysis, Ni/C nano-material, core-shell structure, heterogeneous catalysis, phenylacetylene hydrogenation
PDF全文下载地址:
点我下载PDF
