摘要/Abstract
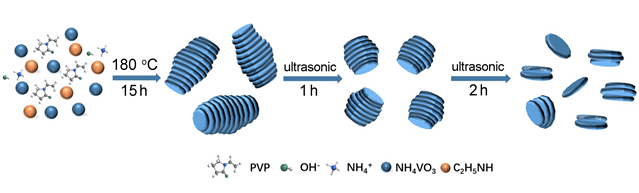
钠离子电池因地壳中丰富的钠资源以及金属钠与金属锂之间具有相似的物化性质等特点,成为后锂时代电池的候选者之一,然而较大的钠离子半径影响了其体系的动力学性能及离子迁移速率,因此寻找合适的电极材料成为其发展的关键.二硫化钒作为过渡金属硫属化合物,具有类石墨烯的层状结构,为钠离子的储存提供了足够的空间,同时其出色的导电性能也为其作为高性能钠离子电池的电极材料提供了保证.利用水热法与超声剥离法,可控制备出三种堆叠密度不同的铜钱状二硫化钒(VS2-Long、VS2-Middle、VS2-Short),并将其用于储钠性能研究.结果表明,堆叠程度最低的VS2-Short因其形貌结构特点而拥有较多的活性位点及较高结构稳定性,在100 mA·g-1的电流密度下,循环300圈后容量高达410 mAh·g-1;电流密度为2000 mA·g-1,可逆容量仍高达333 mAh·g-1.此外,还研究了二硫化钒作为钠离子电池电极材料的储能机制,通过非原位X射线粉末衍射(XRD)及透射电子显微镜(TEM)观测发现:放电过程中,电压在2.5~1.0 V发生嵌钠反应生成NaxVS2,之后逐渐开始转化反应生成Na2S和V;充电时Na2S和V转化生成NaxVS2,并最终脱钠生成VS2,即在0.2~2.5 V间VS2表现为嵌入转化的储钠机制.
关键词: 钠离子电池, 过渡金属硫属化合物, 二硫化钒, 电极材料
Sodium ion batteries (SIBs) have become one of candidates for post-lithium batteries due to the rich sodium resources and the similar physico-chemical properties between sodium and lithium, while the larger sodium ion radius affects the kinetic properties and ion mobility of the sodium ion batteries system, so finding the right electrode material has become the key to develop SIBs. Vanadium Disulfide (VS2) as a typical family member of transition metal chalcogenides (TMCs) has the graphene-like layered structure and excellent electrical conductivity, which provides sufficient space for the storage of sodium ions and ensures its high performance as anode for SIBs. In this work, we used the combination of hydrothermal method and ultrasonic stripping method to prepared three different Coin-like VS2 (VS2-Long, VS2-Middle, and VS2-Short) for sodium storage research. The results show that Coin-like VS2-Short (VS2-S) with the lowest stacking degree can expose more active sites and has a more stable structure so that it has a high capacity of 410 mAh·g-1 after 300 cycles at 100 mA·g-1 and a high rate capability of 333 mAh·g-1 even at 2000 mA·g-1. In addition, we also studied the mechanism of vanadium disulfide as electrode material of sodium ion batteries by using the ex-situ X-ray diffraction (XRD) and transmission electron microscopy (TEM). During discharge process, sodium ion was inserted into the layer of VS2 resulting in NaxVS2 at the voltage of 2.5~1.0 V, and then, NaxVS2 convert to sodium sulfide and vanadium between the voltage of 1.0~0.2 V, on the opposite charging process, sodium sulfide with vanadium will convert to NaxVS2 firstly and then vanadium disulfide will appeared again with the sodium ion deserted from the NaxVS2. This means that vanadium disulfide appears to be an insertion-conversion mechanism between 0.2~2.5 V.
Key words: sodium ion batteries (SIBs), transition metal chalcogenides (TMCs), VS2, electrode material
PDF全文下载地址:
点我下载PDF
