摘要/Abstract
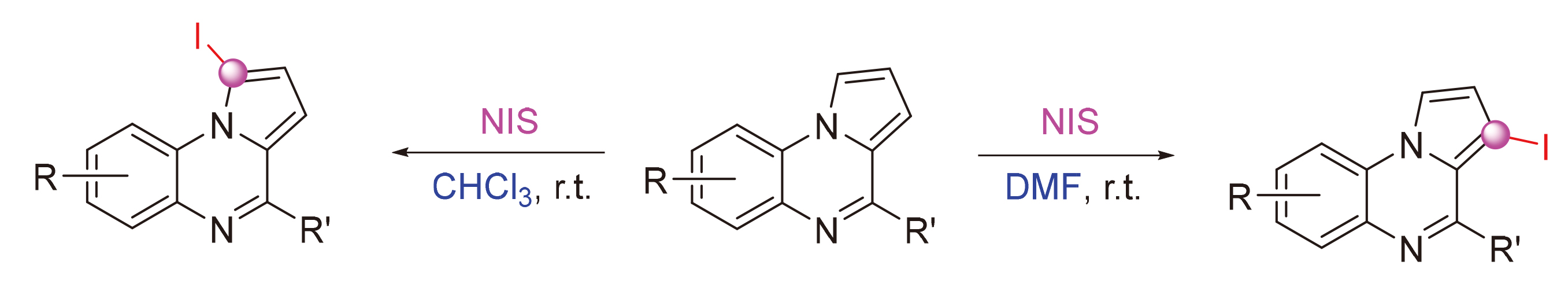
开发了一种溶剂介导吡咯并[1,2-a]喹喔啉和N-碘代丁二酰亚胺(NIS)的区域选择性C—H碘化, 分别以CHCl3和N,N-二甲基甲酰胺(DMF)为溶剂, 选择性地生成了1-碘吡咯并[1,2-a]喹喔啉和3-碘吡咯并[1,2-a]喹喔啉. 此外,吡咯并[1,2-a]喹喔啉与N-溴代丁二酰亚胺(NBS)的溴代反应得到主要产物为1,3-二溴吡咯并喹喔啉. 该方法具有反应条件简单温和、区域选择性好、底物范围广、克级合成等特点. 此外,还通过钯和碘催化的C—X (X=C, S)键形成反应研究了卤化吡咯并[1,2-a]喹喔啉产物的进一步转化.
关键词: 溶剂介导, 区域选择性, 吡咯并[1,2-a]喹喔啉, C—H键碘代
A solvent mediated regioselective C—H iodination of pyrrolo[1,2-a]quinoxaline with N-iodo-succininide (NIS) has been developed. 1-Iodopyrrolo[1,2-a]quinoxalines and 3-iodopyrrolo[1,2-a]quinoxalines could be selectively synthesized by using CHCl3 and N,N-dimethylformamide (DMF) as solvents, respectively. Furthermore, 1,3-dibromopyrroloquinoxalines were obtained as the main products by the bromination of pyrrolo[1,2-a]quinoxaline with N-bromo-succinimide (NBS). The method features simple and mild reaction conditions, good regioselectivity, broad substrate scope, and gram-scale synthesis. In addition, the further transformation of halogenated pyrrolo[1,2-a]quinoxaline products has also been investigated through palladium and iodine catalyzed C—X (X=C, S) bond formation reactions.
Key words: solvent-mediated, regioselectivity, pyrrolo[1,2-a]quinoxaline, C—H bond iodination
PDF全文下载地址:
点我下载PDF
