摘要/Abstract
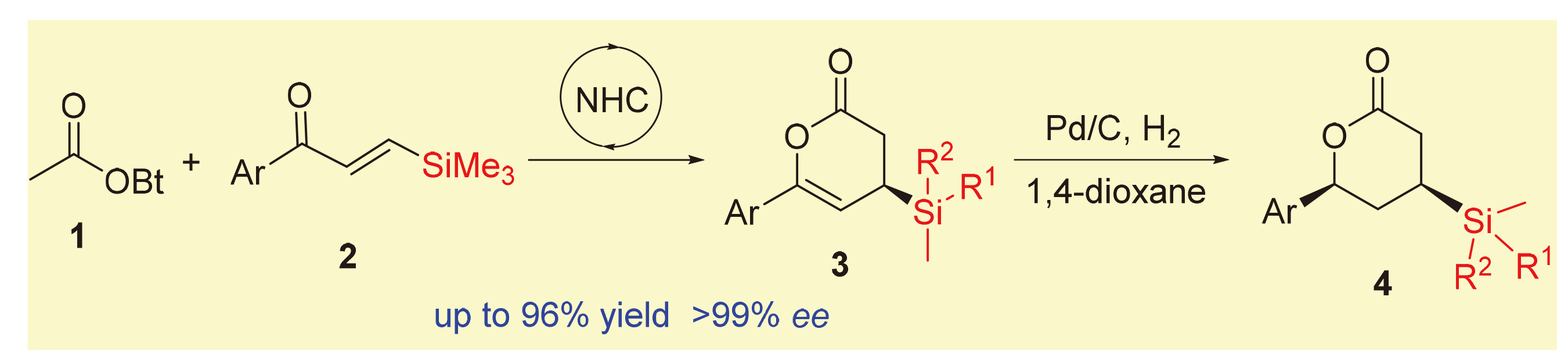
在氮杂环卡宾催化下, 乙酸酯和β-硅基烯酮发生[4+2]环合反应, 可高立体选择性地合成具有潜在应用价值的β-硅基δ-内酯类化合物. 该方法具有底物简单易得、反应条件温和、底物普适性好和操作简单等优点, 且反应规模放大10倍后也得到了优异的产率和对映体选择性. 该反应产物在氢化还原反应中均呈现出优良的实验结果, 可转化为降血脂药Ezetimibe.
关键词: 氮杂环卡宾, 手性有机硅烷化合物, 不对称催化, β-硅基烯酮, 乙酸酯
Asymmetric synthesis of chiral organosilanes with a potential application was realized by N-heterocyclic carbene- catalyzed [4+2] annulation of acetates and β-silyl enones. This strategy exhibits easy to obtain raw materials, mild reaction conditions, good substrate tolerance, simple operation and so on. Notably, excellent yield and enantioselectivity were also observed with expanding the reaction scale by 10 times. The target product showed excellent results in hydrogenation reduction. Moreover, the hypolipidemic drug ezetimibe was obtained through the synthetic transformation of the resulting products.
Key words: N-heterocyclic carbene, chiral organosilanes, asymmetric catalysis, β-silyl enone, acetate
PDF全文下载地址:
点我下载PDF
