摘要/Abstract
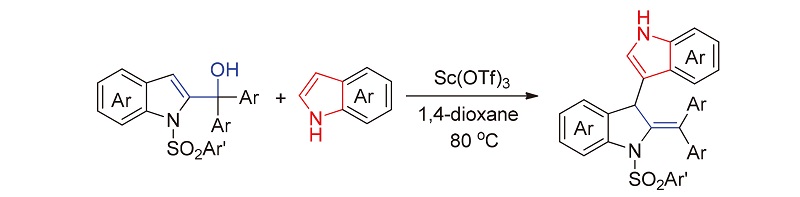
报道了一类新颖的三氟甲基磺酸钪催化的吲哚-2-甲醇的去芳构化反应. 该反应利用吲哚-2-甲醇衍生物在酸性催化下发生极性翻转的特性, 将其吲哚环3-位的亲核中心转变为亲电位点, 实现与另一分子吲哚发生偶联反应, 合成了一系列具有环外双键结构的3,3'-双吲哚衍生物, 产率中等到优秀. 其中N-磺酰基团的强诱导作用和大位阻效应是吲哚-2-甲醇的吲哚环发生去芳构化的关键因素. 基于实验结果及文献报道, 提出了可能的反应机理, 其中涉及吲哚-2-甲醇衍生物的去羟基化和亲核加成等. 此外, 该反应具有高官能团兼容性、条件温和、操作简便等优点.
关键词: 极性翻转, 去芳构化反应, 吲哚-2-甲醇衍生物, 3,3'-双吲哚衍生物
A new Sc(OTf)3-catalyzed dearomatization of indole-2-methanols is reported. By using the characteristics of umpolung of the preformed indole-2-methanols in the presence of acid catalysts, its nucleophilic center at 3-position of indole ring could be transformed into the electrophilic site, thereby realizing the coupling reaction with another molecule indoles, which led to the synthesis of a series of 3,3'-bisindoles with exocyclic double bond unit in moderate to excellent yields. Among them, the strong induction and large steric effects of N-sulfonyl group are the key to the dearomatization of indole ring from indole-2-methanols. Based on the experimental results and literature reports, the possible reaction mechanism is proposed, which involves the dehydroxylation and nucleophilic addition of indole-2-methanol derivatives. In addition, this protocol features high functional group compatibility, mild conditions and simple operation.
Key words: umpolung, dearomatization, indole-2-methanols, 3,3'-bisindoles
PDF全文下载地址:
点我下载PDF
