摘要/Abstract
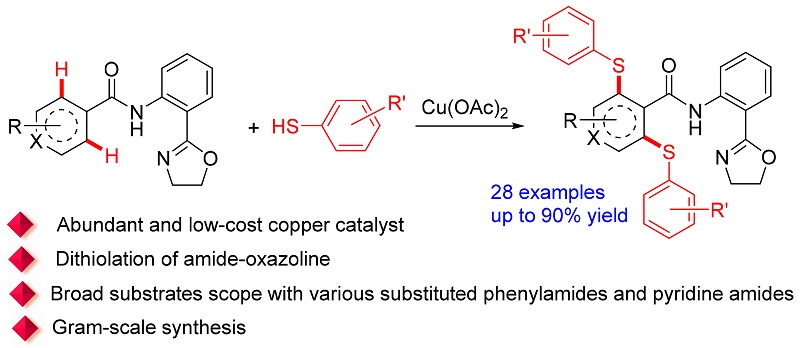
以酰胺-噁唑啉为辅助基团, 在廉价的醋酸铜促进下, 实现了酰胺衍生物C(sp2)—H键与芳基硫醇S—H键的脱氢偶联反应; 以中等到优秀的产率(最高可达90%)简单高效地合成了一系列双硫化的酰胺衍生物. 值得一提的是, 底物范围并不局限于各种取代苯基酰胺化合物, 吡啶基酰胺化合物也可以兼容. 该反应的特点是: 金属廉价、底物范围广、反应条件温和、无需外加配体、空气作为氧化剂、区域选择性好(仅酰胺基团邻位的C—H键发生反应, 而噁唑啉基团邻位的C—H键不发生反应); 此外, 克级规模的反应表明了其在合成中的实用性.
关键词: 铜, C—H键, 硫代化, 酰胺-噁唑啉, 芳基硫醇
An efficient copper-mediated dithiolation of C(sp2)—H bonds with aryl thiols was achieved by using amide-oxazo- line as directing group. This strategy gives a variety of functionalized thioethers in moderate to excellent yields (up to 90%) in simple and efficient way. Importantly, the substrate scope is not limited to various substituted phenylamides, and diverse pyridine amides are also compatible. Furthermore, the protocol has been successfully implemented for the gram-scale synthesis as well.
Key words: copper, C—H bond, thiolation, amide-oxazoline, aryl thiols
PDF全文下载地址:
点我下载PDF
