摘要/Abstract
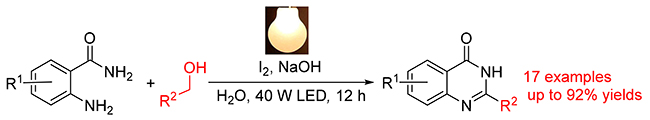
通过在水相中可见光下2-氨基苯甲酰胺与苄醇氧化制备喹唑啉酮的环化反应, 应用廉价易得、操作简单的单质碘作为光催化剂, 在室温下反应获得较好收率的产物. 目标产物的最高产率可达92%, 为喹唑啉酮类化合物的合成提供了一种绿色经济的方法. 运用此策略合成的N-(2-氟-5-甲基苯基)-6-(2,2,2-三氟乙氧基)蝶啶-4-胺对肿瘤细胞具有明显的抑制活性.
关键词: 可见光, 单质碘, 喹唑啉酮, 氧化剂, 催化剂
A novel visible-light-introduced reaction for the construction of quinazolinone derivatives via radical cyclization of 2-aminobenzamides with benzyl alochols under water phase has been developed. The reaction has been achieved in high yield under mild conditions by using I2 as photocatalyst, which is cheap, available and easy to handle. A variety of quinazolinones were obtained in yields up to 92%. It might provide a promising protocol for the synthesis of quinazolinone derivatives. Its application was performed by the synthesis of N-(2-fluoro-5-methylphenyl)-6-(2,2,2-trifluoroethoxy)pteridin-4-amine, which displayed significant inhibitory activity.
Key words: visible light, Iodine, quinazolinone, oxidant, catalyst
PDF全文下载地址:
点我下载PDF
