摘要/Abstract
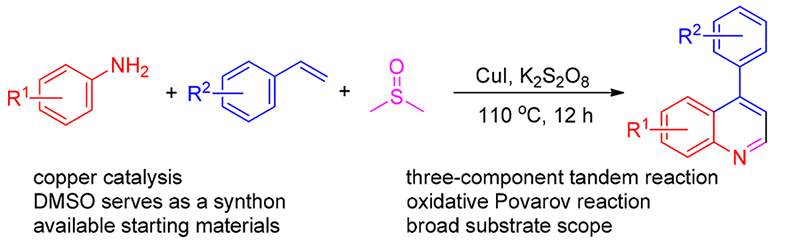
以苯胺、苯乙烯为原料, 二甲基亚砜(DMSO)为C1合成子, 开发了一种铜催化三组分反应合成喹啉衍生物的方法. 机理研究表明, 反应先形成亚胺中间体, 再发生Aza-Diels-Alder反应. 该方法具有高效、环境友好和底物适用范围广等特点.
关键词: 铜催化, 多组分反应, 喹啉衍生物, Aza-Diels-Alder反应
A tandem three-component reaction for the synthesis of quinolines from anilines, styrene and dimethyl sulfoxide (DMSO) has been developed. Dimethyl sulfoxide (DMSO) served as one-carbon synthon and solvent. The mechanism studies revealed that imine intermediate was involved and inverse electron demand Aza-Diels-Alder reaction was occurred. This method is featured by environmentally benign, good functional group tolerance and good to excellent yield.
Key words: copper-catalyzed, multi-component reaction, quinoline derivatives, Aza-Diels-Alder reaction
PDF全文下载地址:
点我下载PDF
