摘要/Abstract
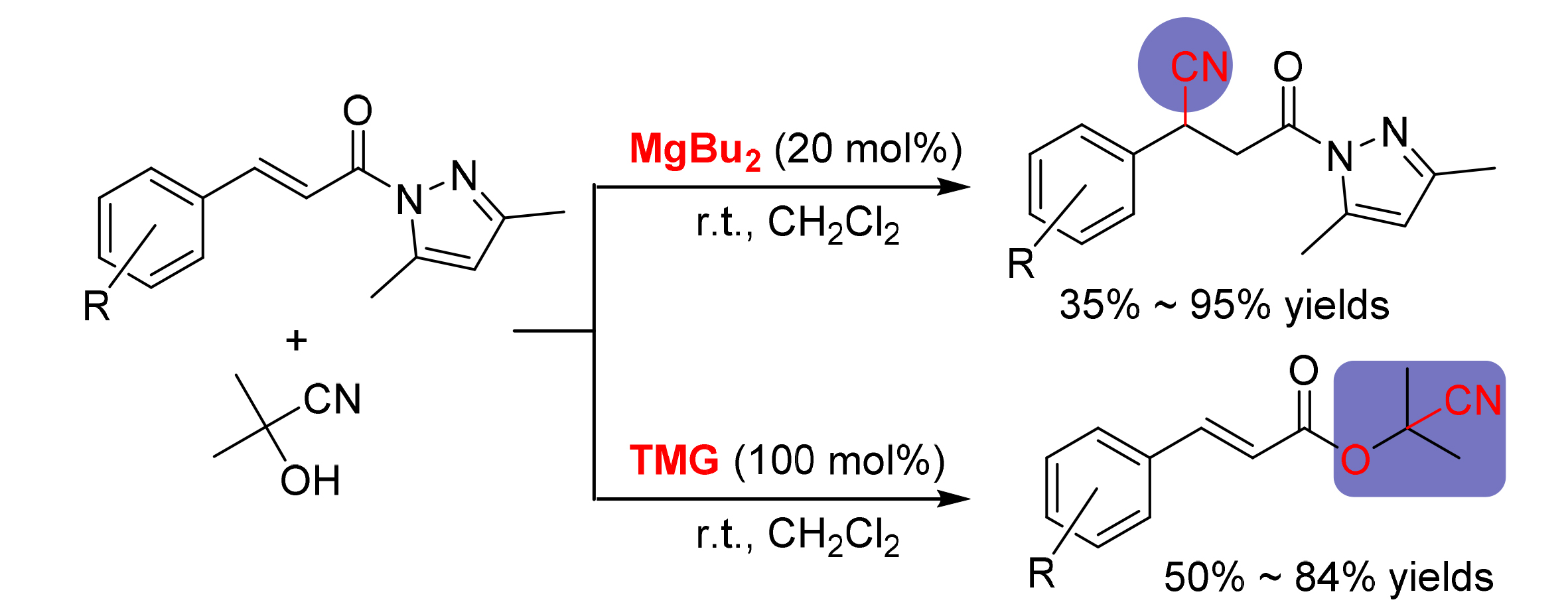
氰基化合物是一类具有重要价值的有机物.研究了以丙酮氰醇为反应试剂,以取代的3,5-二甲基-N-α,β-不饱和酰基吡唑为底物时发生在不同位点的两种反应.研究结果表明,芳香取代的3,5-二甲基-N-α,β-不饱和酰基吡唑为底物时,使用不同的碱性催化剂会发生两种不同类型的反应:MgBu2存在时体系发生Michael加成反应,产物收率最高可达95%;在四甲基胍(TMG)存在时体系发生酰胺的醇解反应,生成β-取代苯基丙烯酸氰醇酯,收率最高达84%.脂肪取代的3,5-二甲基-N-α,β-不饱和酰基吡唑与丙酮氰醇在MgBu2或TMG存在下均发生Michael加成反应,产率最高为99%.讨论了使用不同碱性催化剂时的反应机理.
关键词: 丙酮氰醇, 3,5-二甲基-N-α,β-不饱和酰基吡唑, Michael加成反应, β-取代苯基丙烯酸氰醇酯
Cyano compounds are a class of organic compounds with great value. Two different kinds of reactions have been reported using acetone cyanohydrin as reagent and substituted 3,5-dimethyl-N-α,β-unsaturated acyl pyrazole as substrate. The reaction pathway depends on the basic catalysts used when aromatic substituted 3,5-dimethyl-N-α,β-unsaturated acyl pyrazole was used as the substrate. Michael addition reaction occurred in the presence of MgBu2 with the product yield up to 95%, while alcoholysis reaction of amide occurred in the presence of 1,1,3,3-tetramethylguanidine (TMG), producing β-substituted phenyl cyanoacrylates with 84% yield. However, fatty substituted 3, 5-dimethyl-N-α,β-unsaturated acyl pyrazole and acetone cyanohydrin underwent Michael addition reaction in the presence of MgBu2 or TMG, the product yield was up to 99%. The possible reaction mechanism when using different basic catalysts was discussed.
Key words: acetone cyanohydrin, 3,5-dimethyl-N-α,β-unsaturated acyl pyrazole, Michael addition, β-substituted phenyl cyanoacrylate
PDF全文下载地址:
点我下载PDF
