摘要/Abstract
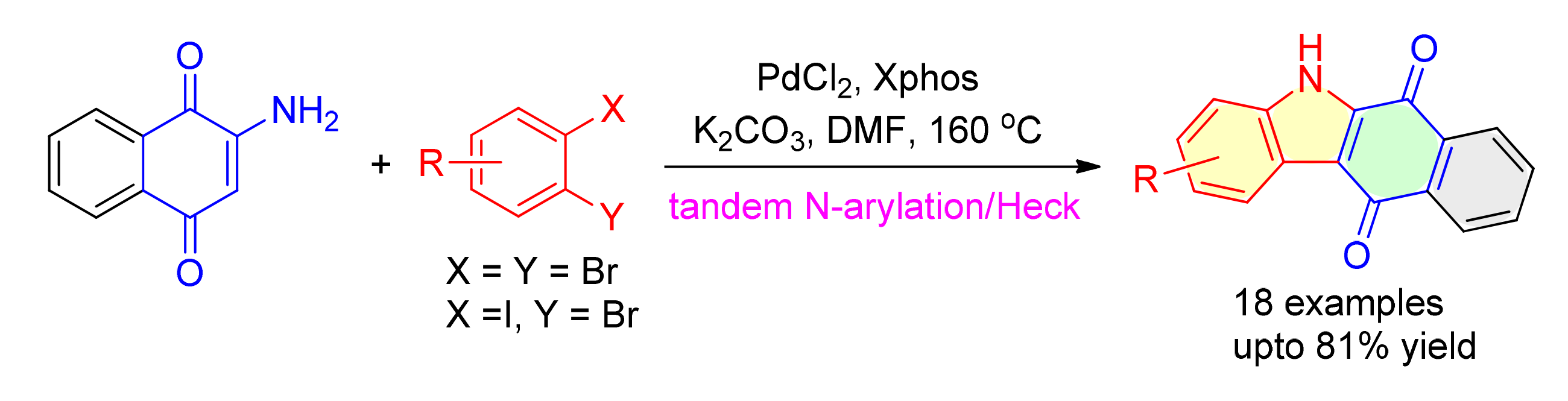
以2-氨基萘醌和邻二溴苯为起始原料,详细探讨了钯催化"一瓶法"合成咔唑醌类化合物的方法.研究结果表明,以氯化钯为催化剂、2-二环己基膦-2,4,6-三异丙基联苯为配体、碳酸钾为碱、N,N-二甲基甲酰胺为溶剂,于160℃下反应72 h获得81%产率的N—H/C—H双芳基化串联反应产物.在此标准条件下,通过改变邻二卤代芳烃的结构,研究了该合成方法的适用范围与局限性,合成得到的系列咔唑醌衍生物经1H NMR和13C NMR结构表征.
关键词: 钯催化, 咔唑醌, 胺化, 芳基化, 串联反应
Starting from 2-aminonaphthalene-1,4-dione and o-dibromoarene, palladium-catalyzed one-pot synthesis of carbazolequinone was examined in detail. With PdCl2 as the catalyst, 2-dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl (Xphos) as ligand and K2CO3 as base in N,N-dimethylformamide (DMF) at 160℃ for 72 h, the annulation reaction afforded the corresponding product by N-H/C-H double arylation process in 81% yield. With different o-dihaloarenes, a series of carbazolequinone derivatives were synthesized to examine the scope and limitation of the above method, and the structure of products was characterized by 1H NMR and 13C NMR spectra.
Key words: palladium-catalyzed, carbazolequinones, amination, arylation, tandem reaction
PDF全文下载地址:
点我下载PDF
