摘要/Abstract
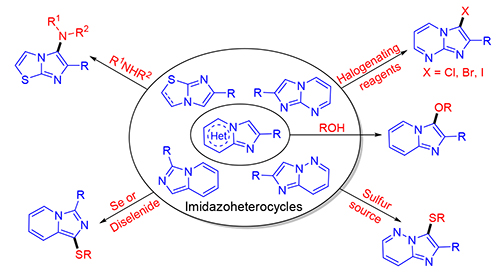
通过无过渡金属催化的C—H官能团化反应构筑C—杂原子键的研究发展迅速,已经成为一种合成高度官能团化天然产物或生理活性分子的绿色、高效的合成策略,包括氨基化、烷氧基化、巯基化、硒基化、卤化化合物等.特别是,咪唑并杂环的C—杂原子化反应被视为最重要的一类反应,因为向杂环分子中引入杂原子基团可以产生一类新的生物活性化合物.重点介绍了近几年无过渡金属催化的在咪唑并杂环上形成C—杂原子键的研究进展,进一步阐述该类反应的机理.
关键词: 杂原子, 咪唑并杂环, 无过渡金属, 反应机理
Recently, the direct incorporation of heteroatom into imidazole-fused heterocycles through transition metal-free C-H functionalization has rapidly been advanced and become an eco-friendly synthetic tool for the synthesis of functionalized natural or bioactive molecules such as (hetero)arenes, olefins, carbonyl compounds. In particular, the C-H functionalization of imidazole-fused heterocycles has been considered to be the most important since it can lead to a new class of biologically active compounds. The recent progress in the incorporation of heteroatom into imidazole-fused heterocycles through transition metal-free C-H functionalization is introduced, and their mechanisms from a new perspective are also elaborated.
Key words: heteroatom, imidazole-fused heterocycles, transition metal-free, reaction mechanism
PDF全文下载地址:
点我下载PDF
