摘要/Abstract
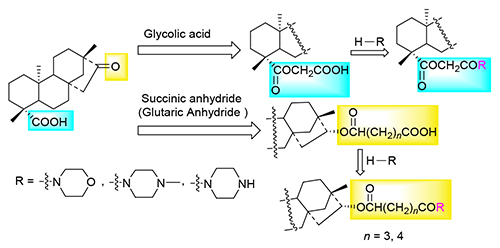
以异甜菊醇为先导化合物,通过连接片段分别在C-16和C-19位引入吗啉和哌嗪结构,并对D环进行结构改造,设计合成了15种未见文献报道的异甜菊醇衍生物,并观察其对人皮肤鳞癌细胞(Colo-16)和人肺腺癌细胞(A549)抑制作用.生物活性结果显示,有4种化合物对人皮肤鳞状癌细胞(Colo-16)的抑制活性明显优于阳性对照物5-氟尿嘧啶.同时观察其对大肠杆菌(E.coli 1924)、金黄色葡萄球菌(S.aureus 4220)、变形链球菌(S.mutans 3289)、耐甲氧西林金黄色葡萄球菌(MRSA 3167)和耐喹诺酮金黄色葡萄球菌(QRSA 3505)的抑制作用,结果显示,有6种化合物对S.mutans 3289的最小抑菌浓度(MIC)为8 μg/mL,与阳性对照物氯霉素相当;16β-O-[5-氧亚基-5-(4-甲基-1-哌嗪基)-戊酰基]-19-贝叶酸乙酯(IS-12b)对MRSA 3167的最小抑菌浓度(MIC)为8 μg/mL,与阳性对照物氯霉素相当.
关键词: 异甜菊醇, 细胞毒活性, 抑菌活性
Fifteen novel isosteviol derivatives were designed and synthesized by introducing morpholine and piperazine moieties into C-16 and C-19 position through connecting fragments, and the D-ring structure was modified. The inhibitory effects on Colo-16 and A549 were observed, the results showed that the inhibitory activities of 4 compounds on Colo-16 were significantly better than the positive control of 5-fluorouracil. The inhibitory effects on E. coli 1924, S. aureus 4220, S. mutans 3289, MRSA 3167 and QRSA 3505 were also observed, the results showed that the minimum inhibitory concentration (MIC) of 6 compounds on S. mutans 3289 was 8 μg/mL, which was comparable to the positive control chloramphenicol. The MIC of 16β-O-(5-oxo-5-(4-methyl-1-piperazinyl)-valeryl)]-19-beyeran acid ethyl ester (IS-12b) on MRSA 3167 was 8 μg/mL, which was comparable to the positive control chloramphenicol.
Key words: isosteviol, cytotoxic activity, antibacterial activity
PDF全文下载地址:
点我下载PDF
