摘要/Abstract
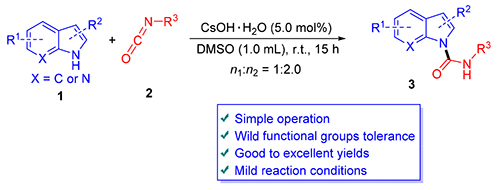
以5.0mol% CsOH·H2O作为催化剂,吲哚衍生物和芳(烷)基异氰酸酯发生N-酰胺化反应,以较高产率制备了一系列吲哚-1-芳(烷)基甲酰胺化合物.该方法对不同的吲哚衍生物和芳(烷)基异氰酸酯均具有较好的适用性,反应均能以较高的产率获得相应的目标产物.与已有方法相比,本方法具有反应条件温和、底物适用范围广、操作简便以及产率高等优点,为吲哚-1-芳(烷)基甲酰胺化合物的制备提供有效的路径.
关键词: 铯催化N-酰胺化, 吲哚-1-甲酰胺, 吲哚衍生物, 芳(烷)基异氰酸酯
A protocol for the synthesis of a series of indole-1-carboxamides was reported via the N-arboxamidation of indole derivatives with aryl (alkyl) isocyanates catalyzed by 5.0 mol% cesium hydroxide monohydrate (CsOH·H2O). This method is suitable for different indole derivatives and aryl (alkyl) isocyanates, for giving corresponding products in excellent yields. Compared to the reported methods, this protocol has the advantages of mild reaction conditions, wild functional group tolerance, simple operation and excellent yields. An efficient route to indole-1-carboxamides is previded.
Key words: cesium-catalyzed N-arboxamidation, indole-1-carboxamides, indole derivatives, aryl (alkyl) isocyanates
PDF全文下载地址:
点我下载PDF
