摘要/Abstract
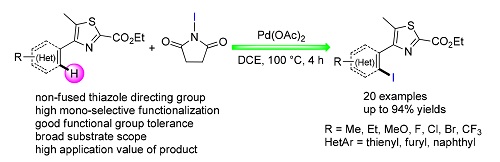
发展了一种钯催化,4-芳基噻唑化合物邻位C(sp2)-H键碘化的反应体系.通过定位基筛选以及反应条件优化得到最高效的单选择性邻位碘化反应条件,并应用于一系列含不同取代基的4-(2-碘代芳基)噻唑的合成中.该催化体系呈现出良好的底物适应性.通过简单的后续衍生化即可将碘化产物转化为多种基团取代产物,为该方法学研究提供了更高的应用价值.通过氘代动力学研究以及自由基抑制实验,提出了合理的催化循环机理.
A palladium-catalyzed ortho-C(sp2)-H bond iodination of 4-arylthiazoles has been developed. Through screening of directing groups and optimazation of reaction parameters, the most efficient reaction conditions for mono-ortho-position iodination were obtained, which were applied to synthesize a series of 4-(2-iodoaryl)thiazoles with broad scope of 4-aryl-thiazole substrates. Furthermore, the iodine group can be easily transformed into other organic functional groups, which improved the application value of this methodology. At last, plausible mechanism was proposed based on an intermolecular deuterium labeling kinetic experiment and radical inhibition experiments.
Key words: palladium-catalyzed, 4-arylthiazoles, mono-selectivity, C(sp2)-H bond, iodination
PDF全文下载地址:
点我下载PDF
