摘要/Abstract
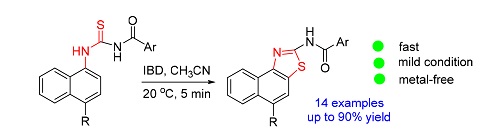
报道了在室温条件下,二醋酸碘苯促进3-[1-(4-取代萘基)]-1-芳酰基硫脲自身关环得到一系列2-芳酰氨基萘并[1,2-d]噻唑衍生物.该方法具有反应条件温和,反应迅速,操作简单,原子利用率高,无需金属催化以及底物范围广等优点.该方法也为萘并[1,2-d]噻唑衍生物的合成提供了新的高效途径.
关键词: 萘并[1, 2-d]噻唑, 硫脲, 二醋酸碘苯, 无金属催化
A novel and efficient approach has been developed to synthesize 2-aroylamino naphtho[1, 2-d]thiazole compounds through the reaction between 3-[1-(4-substituted naphthyl)]-1-aroylthiourea and iodosobenzene diacetate (IBD) under ambient air. A library of naphtho[1, 2-d]thiazole derivatives having a variety of substituents has been synthesized. A plausible reaction pathway has been predicted. This reaction offers a metal-free synthesis, broad substrate scope, easily accessible reactants, excellent regioselectivity, room temperature reaction conditions under ambient air. The reported method is the efficient approach for the synthesis of naphtho[1, 2-d]thiazole derivatives.
Key words: naphtho[1, 2-d]thiazole, thiourea, iodosobenzene diacetate, metal-free
PDF全文下载地址:
点我下载PDF
