摘要/Abstract
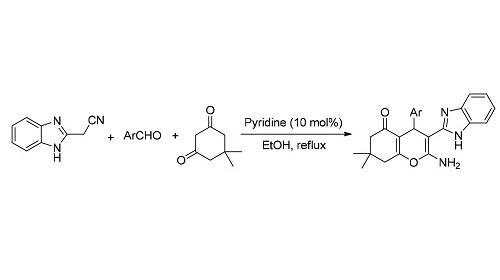
苯并咪唑和色烯类化合物具有广泛的生物活性,同时含有苯并咪唑和色烯骨架的杂环化合物具有较强的Rho激酶抑制活性.然而,目前还缺乏有效的合成方法来制备该类化合物.报道了芳醛、2-氰甲基苯并咪唑和5,5-二甲基-1,3-环己二酮的三组分串联反应,合成了一系列多取代3-苯并咪唑色烯衍生物,产率为48%~89%.该反应以乙醇为溶剂,吡啶为催化剂,在回流条件下反应1~3 h即可得到目标产物.
关键词: 苯并咪唑, 色烯, 多组分反应, 杂环骨架
Benzoimidazole and chromen derivatives exhibit a variety of important biological activities. Chromens incorporating benzoimidazole moiety have high Rho kinase inhibitory activity. However, the effective synthetic method for the preparation of these compounds is rare. The efficient synthesis of new substituted 3-(1H-benzo[d]imidazol-2-yl)-4H-chromens in 48%~89% yields via one-pot, three-component reaction of 2-(1H-benzo[d]imidazol-2-yl)acetonitrile with aromatic aldehydes and 5, 5-dimethylcyclohexane-1, 3-dione was studied. This reaction was carried out in EtOH in the presence of pyridine under reflux conditions. All reactions were completed within 1 to 3 h.
Key words: benzoimidazole, chromen, multicomponent reaction, heterocyclic skeleton
PDF全文下载地址:
点我下载PDF
