摘要/Abstract
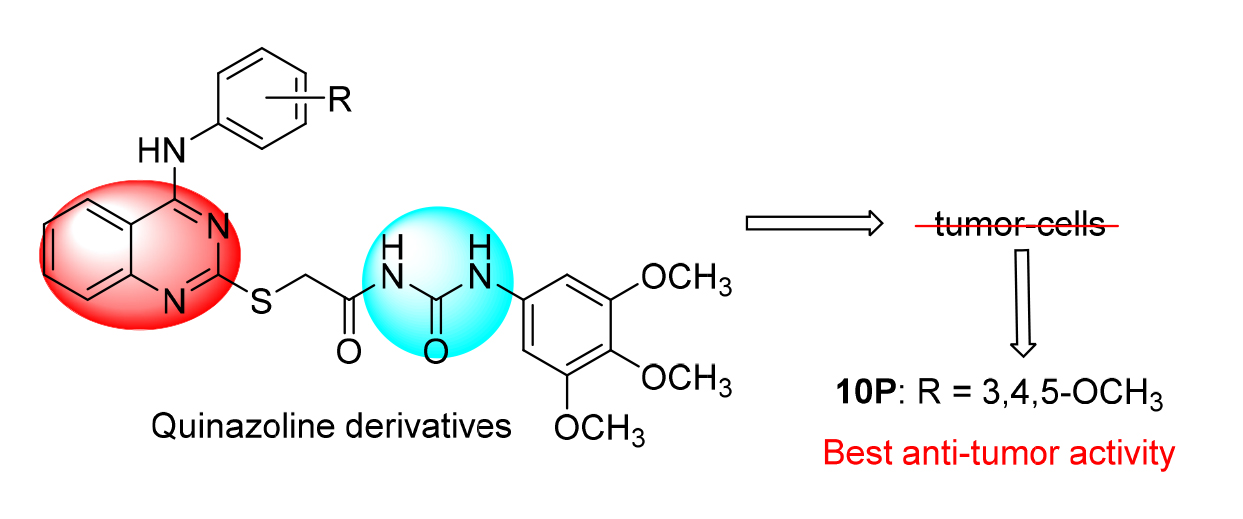
为了寻找新的具有靶向治疗作用的抗肿瘤药物, 设计并合成了一系列新型的含脲砌块的4-氨基喹唑啉类衍生物, 并采用噻唑蓝(MTT)法测定目标化合物对MCF-7(人乳腺癌细胞)、MGC-803(人胃癌细胞)、SW620(人结肠癌细胞)、A549(人肺癌细胞)四种肿瘤细胞的抗肿瘤活性. 结果显示大部分化合物具有较好的抗肿瘤活性, 其中2-((4-((3,4,5-三甲氧基苯基)-氨基)喹唑啉-2-基)-硫代)-N-((3,4,5-三甲氧基苯基)氨基甲酰基)乙酰胺(10p)对MGC-803、SW620和A549三种细胞显示出最好的抗肿瘤活性, IC50值分别为(7.02±0.46)、(6.00±0.78)和(7.04±1.11) μmol?L –1, 其抗肿瘤活性和阳性对照品吉非替尼相当. 分子对接结果显示, 化合物10p能与EGFR很好地结合, 有可能成为潜在的抗肿瘤药物.
关键词: 脲砌块, 4-氨基喹唑啉, 合成, 抗肿瘤活性
In order to find new anti-tumor drugs with targeted therapeutic effect, a series of novel 4-aminoquinazoline derivatives bearing urea moiety were designed, synthesized and evaluated for antitumor activity against four human cancer cell lines of MCF-7, MGC-803, SW620 and A549 using methyl thiazolyl tetrazolium (MTT) assay. Most of the target compounds exhibited excellent anti-tumor activity against the four human tumor cell lines. Among them, 2-((4-((3,4,5-trimethoxyphenyl)- amino)quinazolin-2-yl)-thio)-N-((3,4,5-trimethoxyphenyl)carbamoyl)acetamide (10p) showed the best antitumor activity against MGC-803, SW620 and A549 cancer cell lines with IC50 values of (7.02±0.46), (6.00±0.78) and (7.04±1.11) μmol? L –1, respectively. Its activity was comparable to the positive control of gefitinib. Molecular docking showed that compound 10p could bind well with EGFR, suggesting that it could be a potential antitumor agent.
Key words: urea moiety, 4-aminoquinazoline, synthesis, antitumor activity
PDF全文下载地址:
点我下载PDF
