摘要/Abstract
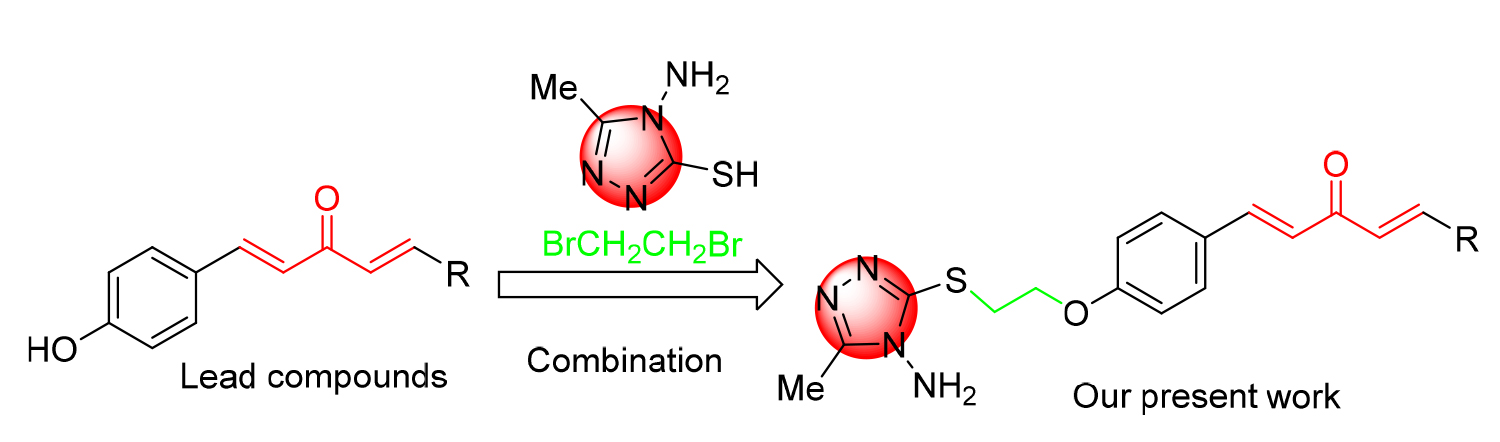
将含硫醇的三唑引入到1,4-戊二烯-3-酮结构中, 合成一系列含硫醚三唑的1,4-戊二烯-3-酮类衍生物, 其结构通过 1H NMR、 13C NMR、HRMS进行表征. 生物活性测试结果表明: 目标化合物对柑橘溃疡病菌(X. citri)、水稻白叶枯病菌(X. oryzae)、烟草青枯病菌(R. solanacearum)都表现出一定的抑制活性. 其中, 化合物F4、F6、F16对柑橘溃疡病菌的EC50值分别为16.3、9.9、15.9 μg/mL, 优于对照药叶枯唑(54.9 μg/mL); 化合物F1、F7、F15对水稻白叶枯病菌的EC50值分别为9.6、19.2、21.3 μg/mL, 优于对照药叶枯唑(69.3 μg/mL); 化合物F3、F6对烟草青枯病菌的EC50值分别为14.2、14.5 μg/mL, 优于对照药叶枯唑(82.6 μg/mL). 通过扫描电镜成像探讨了目标化合物F6对柑橘溃疡病菌(X. Citri)的可能抑菌机制.
关键词: 1,4-戊二烯-3-酮, 硫醚三唑, 生物活性, 扫描电镜
A series of novel 1,4-pentadien-3-one derivatives containing thioether triazole units were synthesized by introducing triazoles bearing thiol groups into the structures of 1,4-pentadien-3-one. The structures of the newly synthesized compounds were assigned via 1H NMR, 13C NMR and HRMS. Bioassays indicated that some of the compounds showed potential antibacterial activities against X. citri, X. oryzae and R. solanacearum. Among them, compounds F4, F6 and F16 demonstrated appreciable inhibitory effect against Xanthomonas axonopodis pv. citri, with half-maximal effective concentration (EC50) values of 16.3, 9.9 and 15.9 μg/mL, which were better than commercial agent bismerthiazol (54.9 μg/mL). Compounds F1, F7 and F15 demonstrated appreciable inhibitory effects against Xanthomonas oryzae pv. Oryzae with EC50 values of 9.6, 19.2 and 21.3 μg/mL, which were better than commercial agent bismerthiazol (69.3 μg/mL). Compounds F3 and F6 also demonstrated appreciable inhibitory effects against Ralstonia solanacearum with EC50 values of 14.2 and 14.5 μg/mL, which were better than commercial agent bismerthiazol (82.6 μg/mL). The possible mechanism of the antibacterial activity of the target compound F6 against Xanthomonas axonopodis was investigated through scanning electron microscopy.
Key words: 1,4-pentadien-3-one, thioether triazole, biological activity, scanning electron microscope
PDF全文下载地址:
点我下载PDF
