摘要/Abstract
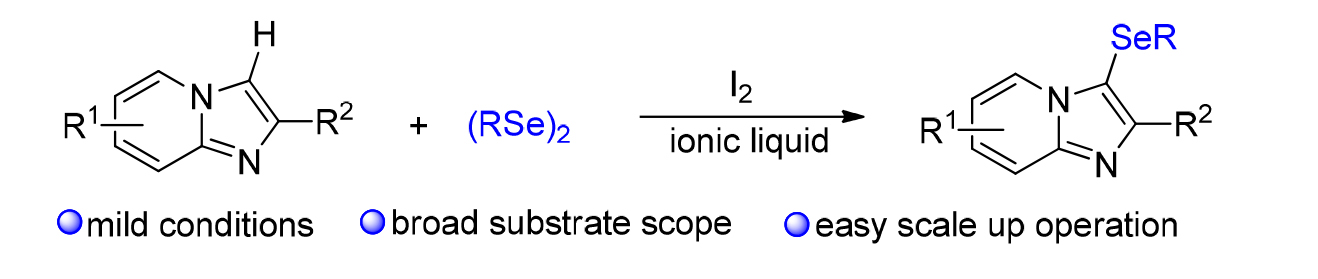
发展了一种环境相对友好的、咪唑并吡啶衍生物和有机硒化合物C-3位的硒化反应, 目标产物能以中等到优的收率获得. 初步机理研究表明, 该硒化反应经历了亲电加成反应机制, 具有反应条件温和、底物范围宽泛、易于放大量生产等特点. 因此, 该策略在合成含氮和含硒分子中具有重要的应用前景.
关键词: 硒化, 咪唑并吡啶, C-3官能化, 亲电机理
An relative eco-friendly protocol for the direct C-3 selenation of imidazo[1,2-a]pyridines with vorious organoselenides has been developed, gaving the desired products in moderate to excellent yields. Preliminary experimental results are consistent with a C-3 electrophilic functionalization mechanism. The features with relative green reaction conditions, broad substrate scope, and easy scale-up operation would make this strategy promising and important for the preparation of nitrogen and selenium containing molecules.
Key words: selenation, imidazo[1,2-a]pyridines, C-3 functionalization, electrophilic mechanism
PDF全文下载地址:
点我下载PDF
