摘要/Abstract
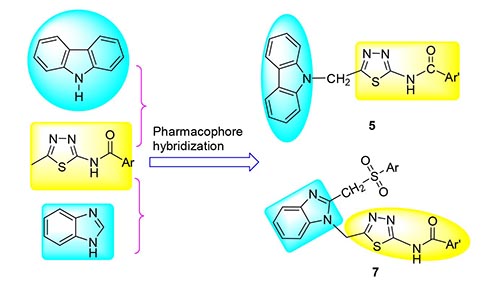
合成了一系列新型含咔唑/苯并咪唑环的2,5-二取代-1,3,4-噻二唑酰胺衍生物.利用IR,1H NMR,13C NMR和元素分析对其进行了结构表征.评价了目标化合物对蛋白酪氨酸磷酸酶1B(PTP1B)和T细胞蛋白酪氨酸磷酸酶(TCPTP)的抑制活性,讨论了结构与活性的关系.实验结果显示,绝大多数化合物对PTP1B的抑制活性超过高度同源的TCPTP的抑制活性,其中2-(9-咔唑基亚甲基)-5-(3-氯苯甲酰氨基)-1,3,4-噻二唑(5c)对PTP1B的抑制活性最高[IC50=(2.43±0.43)μg/mL],2-(9-咔唑基亚甲基)-5-(4-甲基苯甲酰氨基)-1,3,4-噻二唑(5b)和化合物5c对PTP1B的抑制活性均高于阳性对照药物齐墩果酸.对目标化合物5c进行分子对接研究和密度泛函理论(DFT)计算.分子对接结果表明,5c与PTP1B酶通过形成氢键、疏水和π-π等相互作用形成稳定的复合物.
关键词: 1,3,4-噻二唑酰胺, 咔唑, 苯并咪唑, 合成, 蛋白酪氨酸磷酸酶1B(PTP1B)抑制剂, 分子对接, 密度泛函理论(DFT)计算
A series of novel 2,5-disubstituted-1,3,4-thiadiazolamide derivatives containing carbazole/benzimidazole moity were synthesized. Their structures were characterized by IR, 1H NMR, 13C NMR spectra and elemental analysis. All synthesized target compounds were evaluated for the inhibitory activities against protein tyrosine phosphatase 1B (PTP1B) and T-cell protein tyrosine phosphatase (TCPTP). The structure-activity relationship was discussed. The results showed that most of compounds had good inhibitory activity against PTP1B over the highly homologous TCPTP, and 2-(9-carbazolylmethylene)-5-(3-chlorobenzoylamino)-1,3,4-thiadiazole (5c) displayed the highest inhibitory activity against PTP1B[IC50=(2.43±0.43) μg/mL]. The inhibitory activities of 2-(9-carbazolylmethylene)-5-(4-methylbenzoylamino)-1,3,4-thiadiazole (5b) and 5c against PTP1B were higher than that of positive control oleanolic acid. Molecular docking and density functional theory (DFT) calculations of the target compound 5c were performed. The docking result showed that compound 5c and PTP1B enzyme formed a stable complex by hydrogen bonds, hydrophobic and π-π interactions.
Key words: 1,3,4-thiadiazolamide, carbazole, benzimidazole, synthesis, PTP1B inhibitor, molecular docking, density functional theory (DFT) study
PDF全文下载地址:
点我下载PDF
