摘要/Abstract
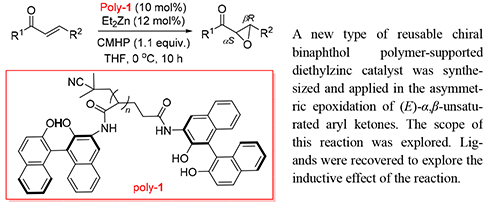
小分子手性催化剂存在着不便于与产物分离且催化剂难以回收重复使用等问题,这使得聚合物负载型的可回收催化剂得到了广泛的关注和研究.本工作成功地将可重复使用的手性联萘酚聚合物负载的二乙基锌催化剂用于(E)-α,β-不饱和芳基酮的不对称环氧化反应中.在对反应条件进行了优化后研究了反应的底物普适性,进一步探索了回收后的催化剂在以上不对称反应中的催化效率.该反应中对映体过量值最高能达到94%.分别对聚合物进行了回收利用实验,结果表明经过多次重复使用的聚[(S)-3-丙烯酰胺基-2,2'-二羟基-1,1'-联二萘]的手性诱导能力并没有明显下降.
关键词: 联萘酚, 手性聚合物, 二乙基锌, 查尔酮, 不对称催化
Most small chiral molecule catalysts are suffered from a rigid process in both product separation and recovery. Therefore more attention has been drawn to the soluble polymer-supported catalyst which could be easily recycled. In this paper, a new type of reusable chiral binaphthol polymer-supported diethylzinc catalyst was synthesized and applied in the asymmetric epoxidation of (E)-α,β-unsaturated aryl ketones. The scope of this reaction was explored. Various (E)-α,β-aryl ketones could be easily prepared in good yield (up to 88%) and high ee value (up to 94%) via this asymmetric epoxidation process. Ligands were recovered to explore the inductive effect of the reaction. Recovery experiments of this binaphthol polymer-supported diethylzinc catalyst were conducted. The results indicate that the asymmetric induction ability of the reclaimed chiral polymer 1,1'-bi-2-naphthol did not decrease significantly.
Key words: 1,1'-bi-2-naphthol, Et2Zn, chiral polymer, asymmetric addition, aromatic ketone
PDF全文下载地址:
点我下载PDF
