摘要/Abstract
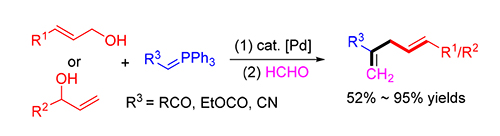
报道了钯催化下酮基稳定的磷叶立德与烯丙基醇一锅法的烯丙化-Wittig反应.研究表明,在5 mol%四(三苯基膦)钯和20 mol%硼酸的共催化下,以52%~95%的收率得到官能化1,4-二烯化合物.该方法还可以进一步拓展到酯基以及氰基稳定的磷叶立德来合成对应的1,4-二烯化合物.
关键词: 稳定磷叶立德, 烯丙基醇, 脱水偶联, 钯催化
A dehydrative cross coupling of ketone-stabilized phosphorus ylides with the readily available allylic alcohols followed by an one-pot Wittig reaction is developed. A range of functional 1,4-dienes could be obtained in 52%~95% isolated yields in the presence of 5 mol% Pd(PPh3) 4 and 20 mol% B(OH) 3. The same method can be extended to ester or nitrile-stabi-lized phosphorus ylides, affording the corresponding 1,4-dienes in moderate yields.
Key words: stabilized phosphorus ylides, allylic alcohols, dehydrative cross couplings, palladium catalysis
PDF全文下载地址:
点我下载PDF
