摘要/Abstract
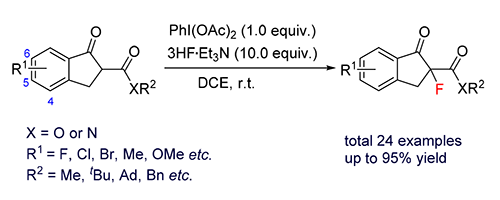
报道了一种构建含氟的β-酮酸酯及β-酮酰胺的亲核氟化反应.该反应采用PhI(OAc)2为氧化剂、3HF·Et3N为氟化试剂,在室温下反应30 min高效构建一系列含有季碳中心的含氟化合物.与传统的亲电氟化反应相比,该方法具有无金属参与、反应时间短、反应条件简单、反应收率高等优点.
关键词: 高价碘, β-酮酸酯, β-酮酰胺, 亲核氟化反应
Herein, a nucleophilic fluorination reaction to construct fluorine-containing β-ketoesters and β-ketoamides is reported. The reaction uses PhI(OAc)2 as oxidant and 3HF·Et3N as fluorinating reagent. It can effectively build a series of fluorochemical compounds containing quaternary carbon center under room temperature reaction conditions for 30 min. Compared with the traditional electrophilic fluorination reaction, this method has the advantages of no metal participation, short reaction time, simple reaction conditions and high reaction yield.
Key words: hypervalent iodine, β-ketoesters, β-ketoamides, nucleophilic fluorination reaction
PDF全文下载地址:
点我下载PDF
