摘要/Abstract
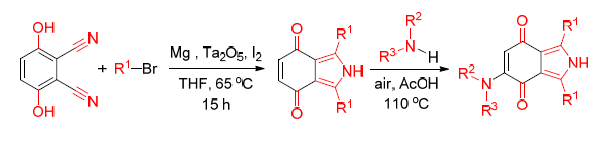
通过Barbier-Grignard型反应,在Ta2O5催化下,2,3-二氰基对苯二酚与溴苯和Mg在四氢呋喃中一锅反应成功制备出含氮杂环化合物1,3-二芳基或烷基-2H-异吲哚-4,7-二酮,该方法新颖、方便且高效,适用于不同芳基和烷基溴代物,最高收率达到96%.此外在一些实验结果的基础上,提出了涉及亲核加成和电子转移的可能的反应机理,利用1,3-二苯基-2H-异吲哚-4,7-二酮(3a)中的醌结构与胺偶联得到了更复杂的结构.
关键词: 异吲哚, Barbier-Grignard型反应, Ta2O5, 氧化偶联
A novel, convenient and efficient protocol to N-heterocyclic derivatives of 1,3-diaryl or diaklyl-2H-isoin-dole-4,7-dione has been developed via the Barbier-Grignard-type reaction. Good to excellent yields up to 96% have been achieved by performing the reaction in one pot using Ta2O5 as the catalyst. The protocol displays good tolerance to different aryl and alkyl bromides. Based on some experimental results, a plausible mechanism involving nucleophilic addition and electron transfer has been proposed. The quinone structure in 1,3-diphenyl-2H-isoindole-4,7-dione (3a) makes it coupling with amine to form more complicated structure.
Key words: isoindole, Barbier-Grignard reaction, Ta2O5, oxidative coupling
PDF全文下载地址:
点我下载PDF
