摘要/Abstract
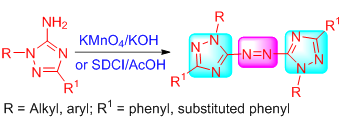
以1,3,5-三取代-1,2,4-三唑类化合物为原料,在KMnO4或二氯异氰尿酸钠(SDCI)作用下,发生自身氧化偶联反应,合成了16个未见文献报道的偶氮桥联的1,3,5-多取代三唑类化合物.产物结构经1H NMR、13C NMR、IR、HRMS及X射线单晶衍射得以证实,并采用生长速率法对所有化合物进行了离体抑菌活性测试.结果表明:在浓度为50 μg/mL时,大部分化合物对所选六种病菌具有一定的抑制活性,其中1,1'-二正丁基-3,3'-二(2''-氯苯基)-5,5'-偶氮二(1,2,4-三唑)(3h)、1,1'-二丙基-3,3'-二(4''-氯苯基)-5,5'-偶氮二(1,2,4-三唑)(3k)对瓜果腐霉菌的抑制率接近三唑酮,而1,1'-二丙基-3,3'-二(2''-氯苯基)-5,5'-偶氮二(1,2,4-三唑)(3g)的抑制率要高于三唑酮.该类化合物反应操作简单、条件温和,对新农药创制及合成具有借鉴意义.
关键词: 三氮唑, 偶氮化合物, 氧化偶联, 抑菌活性
Sixteen bridging ligand compounds of azo with 1,3,5-substitued triazole had been synthesized from 1,3,5-trisub-stituted-1,2,4-triazoles by the oxidative coupling reaction using potassium permanganate or sodium dichloroisocyamirate as the oxidant. The structures of the target compounds were determined by 1H NMR, 13C NMR, IR, MS, HRMS and X-ray diffraction. They were tested for in vitro fungicidal activities by the mycelium growth rate method. The results indicated that most of the title compounds had a certain inhibitory activity at the concentration of 50 μg/mL. Especially, the inhibition rate of 1,2-bis(1-butyl-3-(2-chlorophenyl)-1H-1,2,4-triazol-5-yl)diazene (3h) and 1,2-bis(3-(4-chlorophenyl)-1-propyl-1H-1,2,4-triazol-5-yl)diazene (3k) were close to that of triadimefon when against Pythium aphanidermatum, while the inhibition rate of 1,2-bis(3-(2-chlorophenyl)-1-propyl-1H-1,2,4-triazol-5-yl)diazene (3g) was higher than that of riadimefon. The reactions of these compounds were simple and mild, which was of reference significance to the creation and synthesis of new pesticides.
Key words: triazole, azo compounds, oxidative coupling, fungicidal activities
PDF全文下载地址:
点我下载PDF
