摘要/Abstract
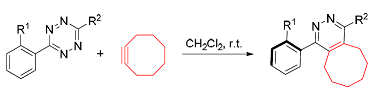
探究了四嗪化合物和环辛炔的[4+2]环加成反应及其在构建轴手性哒嗪骨架中的应用.在不加催化剂的条件下,具有空间位阻的四嗪化合物和大环张力的环辛炔能够在二氯甲烷中发生反电子需求的Diels-Alder反应.反应经历六元桥环过渡态,在温和的条件下脱去一分子氮气,得到具有哒嗪骨架结构的轴手性化合物.该反应能够通过颜色的变化判断反应转化情况,能以很高的产率(95%)得到具有潜轴手性的哒嗪骨架产物.
关键词: 四嗪化合物, 轴手性, 反电子需求Diels-Alder反应, 环辛炔
The application of [4+2] cycloaddition reaction of tetrazine with cyclooctyne in the construction of pyridazine structure with axial chirality was studied. The inverse electronic demand Diels-Alder reaction of tetrazine bearing bulky groups with macrocyclic tension’s cyclooctyne could take place under catalyst-free conditions in dichloromethane. The reaction underwent a six-membered bridged transition state, gently release a molecule of nitrogen to get axial chiral pyridazine structure. The transformation of the reaction can be determined by the change of color. The reaction could get potential axial chiral pyridazine structure with high yiled (95%) under mild conditions.
Key words: tetrazine, axial chirality, the inverse electronic demand Diels-Alder reaction, cyclooctyne
PDF全文下载地址:
点我下载PDF
