摘要/Abstract
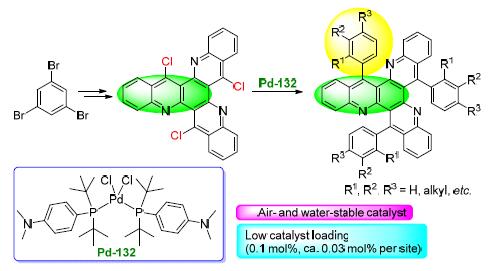
以均三溴苯和邻氨基苯甲酸甲酯为原料,经Buchwald-Hartwig反应、酯基水解和三氯氧磷关环三步合成关键中间体6,12,18-三氯二苯并[b,j]喹啉[2,3-f][1,7]菲罗啉,最后在新型催化剂Pd-132催化下进行Suzuki偶联,高效合成了14个具有代表性的C3-对称-9-芳基吖啶类衍生物.该方法具有催化剂用量低(0.1 mol%,平均到每一个位点仅为0.03 mol%),原料易得,便于推广的特点.
关键词: Buchwald-Hartwig反应, 吖啶, Pd-132, Suzuki偶联
A multiple synthetic strategy is employed to furnish the synthesis of 6, 12, 18-triaryldibenzo[b, j]quinoline[2, 3-f] [1, 7]phenanthroline. The key intermediate 6, 12, 18-trichlorobenzotriquinoline was prepared by Buchwald-Hartwig reaction of 1, 3, 5-tribromobenzene with methyl anthranilate, followed by hydrolysis and POCl3-promoted ring closure reaction. The palladium-catalyzed Suzuki coupling was carried out with a novel catalyst Pd-132 to form fourteen C3-symmetric 9-aryl acridine derivatives. The reactions can be performed at low catalyst loading (0.1 mol%, ca. 0.03 mol% per site).
Key words: Buchwald-Hartwig reaction, acridine, Pd-132, Suzuki coupling
PDF全文下载地址:
点我下载PDF
