摘要/Abstract
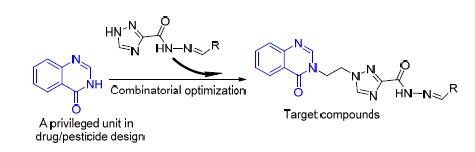
通过三唑酰肼和芳醛的缩合反应,合成了20个含喹唑啉酮-4-酮片段的新型1,2,4-三唑酰腙类化合物,利用核磁共振氢谱、碳谱和高分辨质谱对它们的结构进行了表征.体外抗菌测试表明,绝大部分目标化合物对水稻白叶枯病菌和柑橘溃疡病菌都表现出良好的抑制活性,其中N'-(2-甲氧基亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7q)在100 μg/mL下对上述两种病菌的抑制率均达100%.此外,1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-N'-(4-(三氟甲基)亚苄基)-1H-1,2,4-三唑-3-酰腙(7i)、N'-(2-溴亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7j)、N'-(4-溴亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7l)和N'-(4-甲基亚苄基)-1-(2-(4-氧代喹唑啉-3(4H)-基)乙基)-1H-1,2,4-三唑-3-酰腙(7o)在50 μg/mL下对番茄灰霉病菌的抑制率均超过55%.
关键词: 1,2,4-三唑酰腙, 喹唑啉-4-酮, 合成, 抗菌活性
A total of twenty novel 1, 2, 4-triazole-acylhydrazone derivatives containing the quinazolin-4-one moiety were synthesized via the condensation reaction of triazole hydrazide with various aromatic aldehydes, and fully characterized by 1H NMR, 13C NMR and HRMS spectra. Antimicrobial assays in vitro indicated that most of the target compounds exhibited good antibacterial activities against the pathogenic phytobacteria Xanthomonas oryzae pv. oryzae(Xoo) and Xanthomonas axonopodis pv. citri(Xac). Notably, N'-(2-methoxybenzylidene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhy-drazone)(7q) displayed the inhibition rate of 100% against the above two bacteria at 100 μg/mL. Additionally, 1-(2-(4-oxo-quinazolin-3(4H)-yl) ethyl)-N'-(4-(trifluoromethyl) benzylidene)-1H-1, 2, 4-triazole-3-acylhydrazone (7i), N'-(2-bromobenzyli-dene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7j), N'-(4-bromobenzylidene)-1-(2-(4-oxo-quinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7l), and N'-(4-methoxybenzylidene)-1-(2-(4-oxoquinazolin-3(4H)-yl) ethyl)-1H-1, 2, 4-triazole-3-acylhydrazone (7o) were found to possess the inhibition rate of >55% against the fungus Botrytis cinerea Pers. at 50 μg/mL.
Key words: 1,2,4-triazole-acylhydrazone, quinazolin-4-one, synthesis, antimicrobial activity
PDF全文下载地址:
点我下载PDF
