摘要/Abstract
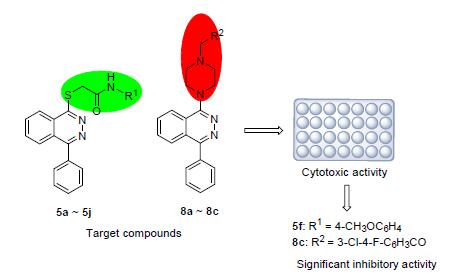
为了找到更有效和更经济的抗肿瘤药物,合成了一系列1-苯基-4-取代酞嗪衍生物,并评估了其体外抗增殖活性.所合成的化合物的结构都通过1H NMR,13 C NMR和HRMS确证.并且通过3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐(MTT)法评估了目标化合物对四种人类癌细胞株的抗肿瘤活性.结果表明:一些化合物具有良好的抗肿瘤活性,特别是N-(4-甲氧基苯基)-2-((4-苯基酞嗪-1-基)硫基)乙酰胺(5f)和N-(3-氯-4-氟苯基)-2-(4-(4-苯基酞嗪-1-基)哌嗪-1-基)乙酰胺(8c)表现出了更好的抗肿瘤活性,对人类食管癌细胞的活性优于5-氟尿嘧啶.IC50值分别为8.13和9.31 μmol·L-1.
关键词: 合成, 酞嗪衍生物, 抗肿瘤活性
In order to find more efficient and economical antitumor drugs, a series of 1-phenyl-4-substituted phthalazine derivatives were synthesized and evaluated for antiproliferative activity in vivo. The structures of the synthesized compounds were confirmed by 1H NMR, 13C NMR and HRMS. The antitumor activity of the target compounds was performed against four cancer cell lines by 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT). The results showed that some compounds had a good antitumor activity, especially, N-(4-methoxyphenyl)-2-((4-phenylphthalazin-1-yl) thio) acetamide (5f) and N-(3-chloro-4-fluorophenyl)-2-(4-(4-phenylphthalazin-1-yl) piperazin-1-yl) acetamide (8c), exhibited better antitumor activities with IC50 values of 8.13 and 9.31 μmol•L-1 against the human esophageal cancer cells, which were superior to 5-fuorouracil.
Key words: synthesis, phthalazine derivative, antitumor activity
PDF全文下载地址:
点我下载PDF
