

中国药科大学 生命科学与技术学院,江苏 南京 211198
收稿日期:2020-12-18;接收日期:2021-03-29;网络出版时间:2021-04-12
基金项目:中国倡议计划(No. CPU2018GY15) 资助
摘要:Kunitz型丝氨酸蛋白酶抑制剂是一类普遍存在的蛋白酶抑制剂,在体内各项生命活动中扮演着重要角色。这类抑制剂结构稳定且富有特色,通常具有一个或几个串联存在的Kunitz结构域,能够以类似底物的方式与丝氨酸蛋白酶结合,从而抑制酶的活性。在功能方面,Kunitz型丝氨酸蛋白酶抑制剂参与凝血和纤维蛋白溶解、肿瘤免疫、炎症调节以及抵抗细菌、真菌感染等过程。文中就Kunitz型丝氨酸蛋白酶抑制剂研究进展作一综述,为新型Kunitz型丝氨酸蛋白酶抑制剂的开发提供研究思路。
关键词:Kunitz结构域蛋白酶抑制剂血凝平衡炎症反应感染免疫调节
Advances of Kunitz-type serine protease inhibitors
Yunyang Liu, Shuai Jiang, Qian Li, Yi Kong


School of Life Science and Technology, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Received: December 18, 2020; Accepted: March 29, 2021; Published: April 12, 2021
Supported by: China Pharmaceutical University, Nanjing, China Initiative Program (No. CPU2018GY15)
Corresponding author: Yi Kong. Tel/Fax: +86-25-83271282; E-mail: yikong@cpu.edu.cn.
Abstract: Kunitz-type serine protease inhibitors are a class of ubiquitous protease inhibitors, which play important roles in various life activities. The structures of such inhibitors are generally stable, and are usually characterized by the presence of one or several Kunitz domains in tandem, which are able to bind to serine proteases in a manner similar to substrate binding, thereby inhibiting enzyme activity. In terms of function, Kunitz-type serine protease inhibitors are involved in processes such as blood coagulation and fibrinolysis, tumor immunity, inflammation regulation, and resistance to bacterial and fungal infections. This article summarizes the advances of Kunitz-type serine protease inhibitors and provides new ideas for the development of novel Kunitz-type serine protease inhibitors.
Keywords: Kunitz domainprotease inhibitorsblood coagulation balanceinflammatory responseinfectionimmune regulation
蛋白酶广泛存在于从细菌到哺乳动物等几乎所有生物体中,在生物体的正常生命活动中扮演重要的角色。蛋白酶抑制剂通过调控蛋白酶的活性来维持机体的稳态,生物体内主要的蛋白酶抑制剂有丝氨酸蛋白酶抑制剂、半胱氨酸蛋白酶抑制剂、天冬氨酸蛋白酶抑制剂和金属蛋白酶抑制剂[1]。丝氨酸蛋白酶抑制剂按其底物可以分为胰蛋白酶样丝氨酸蛋白酶抑制剂(P1位点为带正电荷的Lys/Arg残基),弹性蛋白酶样丝氨酸蛋白酶抑制剂(P1位点为弱疏水Ala/Val残基) 和糜蛋白酶样丝氨酸蛋白酶抑制剂(P1位点为强疏水Phe/Tyr/Leu残基)[2],根据序列同源性、高级结构特征和作用机制,丝氨酸蛋白酶抑制剂可以分为Kunitz、Kazal、Serpin和Mucus家族[3]。Kunitz抑制剂能以类似于底物的方式与丝氨酸蛋白酶以紧密的非共价方式结合,在不改变构象的情况下直接阻断丝氨酸蛋白酶的活性位点从而抑制酶的活性。Kunitz型丝氨酸蛋白酶抑制剂通常含有一个[4]或多个串联的Kunitz结构域[5],在机体的血凝平衡、炎症发生和免疫调节等过程中发挥重要作用。
1 Kunitz型丝氨酸蛋白酶抑制剂的结构特征1930年Kraut发现碱性牛胰蛋白酶抑制剂(Bovine pancreatic trypsin inhibitor,BPTI) 可以抑制激肽酶的活性,1936年Kunitz发现BPTI同样可以抑制胰蛋白酶的活性,这些发现引起了广泛的关注。随后又发现了多种与BPTI同源性高且高级结构类似的多肽和蛋白质,为纪念Kunitz对该研究领域的杰出贡献,把这一类具有共同结构特征的蛋白酶抑制剂称为Kunitz型抑制剂[6]。
这类抑制剂通常具有一个或者多个Kunitz结构域。Kunitz结构域是一类包含50–60个氨基酸残基的多肽,含有4–6个半胱氨酸残基形成2–3对二硫键来保持其高级结构的稳定性,这些半胱氨酸残基之间的空间是保守的,因此4个半胱氨酸残基同样能赋予它们典型的二硫键模式。二级结构主要包括1–2个α螺旋和一对反向平行的β片层。对蛋白酶起抑制作用的部分氨基酸残基序列称为蛋白酶结合环(Loop),该分子表面有2个能够与蛋白酶活性中心结合的Loop从而实现其蛋白酶抑制活性[7] (图 1A)。
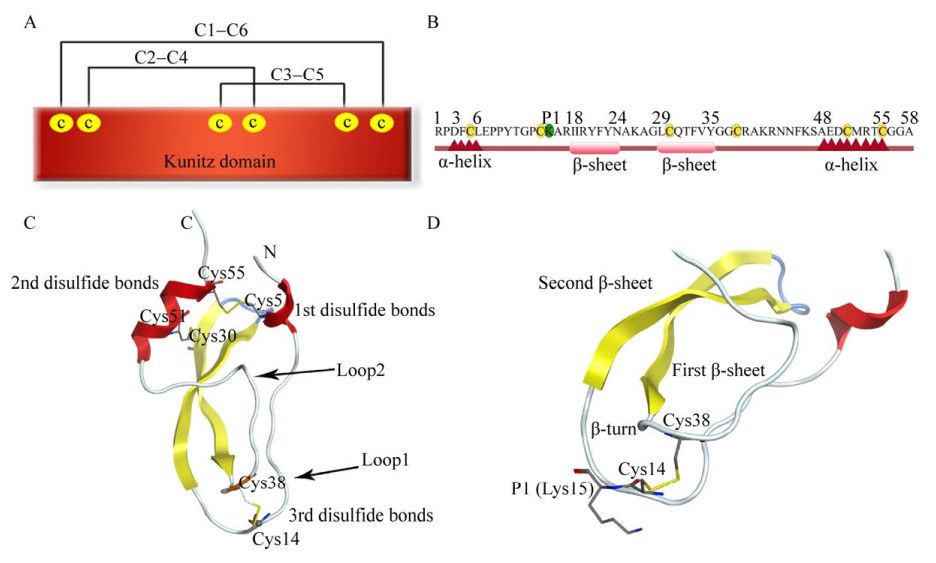 |
| 图 1 Kunitz结构域的结构 Fig. 1 Structure of Kunitz domain. (A) Schematic representation of Kunitz domain inhibitor showing the disulfide bonding pattern of C1–C6, C2–C4, C3–C5. (B) The primary structure of a typical Kunitz inhibitor BPTI. The red triangle represents the α-helix, the light red rounded rectangle represents the β-sheet, the conservative cysteine residues are highlighted in yellow, while the P1 position (Lys15) is in green. (C) Crystal structure of BPTI (PDB: 1BPI) showing three disulfide bonds (in yellow), while the two anti-parallel β-sheets are in yellow and α-helix are in red [40]. (D) The P1 position (Lys15) has an extended side chain that plays a vital role in loop1. |
| 图选项 |
作为Kunitz型丝氨酸蛋白酶抑制剂的典型代表,BPTI由58个氨基酸残基组成,包括6个半胱氨酸形成3对二硫键Cys5 (C1)-Cys55 (C6)、Cys14 (C2)-Cys38 (C4)、Cys30 (C3)-Cys51 (C5),其中前两对二硫键维持该结构域高级结构的保守性,第3对二硫键维持结合位点的稳定性[7]。结构中存在两个α螺旋,C末端的α螺旋比N末端的α螺旋更长更规则,结构中还存在一对反向平行的β片层[8] (图 1B)。该分子具有两个Loop,分别为残基11TGPCKARIIR20和残基34VYGGC38[9],BPTI主要依靠这两个Loop与胰蛋白酶进行广泛的相互作用。Loop1上P1位点赖氨酸残基(Lys15) 可以插入到丝氨酸蛋白酶的S1位点中,是决定抑制剂特异性的关键氨基酸。Asn13、Tyr17和Tyr18可以稳定和反应位点有关的Loop1[10-11]。典型的碱性Kunitz型丝氨酸蛋白酶抑制剂BPTI结构见图 1C和1D[40]。
2 Kunitz型丝氨酸蛋白酶抑制剂的功能丝氨酸蛋白酶抑制剂在生物体中分布广泛,近年来受到科研工作者的高度关注。它们在脊椎动物和无脊椎动物的许多生物学过程中承担着至关重要的作用。
2.1 参与凝血和纤溶过程目前鉴定得到的多种参与凝血的Kunitz型丝氨酸蛋白酶抑制剂大多来源于毒蛇、蜱虫、寄生虫、海葵等动物以及一些豆科植物。内源性蛋白酶抑制剂的单一Kunitz结构域,由于其独特的丝氨酸蛋白酶抑制能力,同样能参与凝血级联过程。这些抑制剂通过调节凝血酶[27]、凝血因子Ⅺ (Factor Ⅺ,FⅪ)[24]、凝血因子Ⅹ (Factor Ⅹ,FⅩ)[26]和纤溶酶[31]等的产生来干扰凝血级联和纤溶过程。参与血凝平衡过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质详见表 1。
表 1 参与血凝平衡过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质Table 1 Origins and properties of Kunitz type serine protease inhibitors involved in the blood coagulation homeostasis
| Organism | Peptide/ protein | Physical and chemical properties | Enzymes inhibited | References |
| Human | PN2KPI | 57 aa | FXIa (Ki=0.81 nmol/L); trypsin (Ki=0.03 nmol/L) | [13] |
| Human | TFPI | MW: 43 kDa | FXa (KD=20.2 nmol/L); TF-FVIIa | [17, 19] |
| Bungarus fasciatus | Fasxiator | MW: 7 kDa | FXIa (IC50=1.5 μmol/L); chymotrypsin | [12, 21] |
| Bungarus flaviceps | Flavikunin | MW: ~7 kDa | Plasmin (IC50=0.48 μmol/L); trypsin | [20] |
| Ixodes ricinus | Ir-CPI | – | PK; FXIIa; FXIa | [24] |
| Simulium vittatum | Simukunin | 83 aa, MW: 9.63 kDa pI: 9.93 | FXIa (IC50=5.20 nmol/L); elastase (KD=0.4 nmol/L, IC50= 4.90 nmol/L); FXa (KD= 3.07 nmol/L) | [25] |
| Ixodes scapularis | Ixolaris | – | FXa; FX | [26] |
| Boophilus microplus | Boophilin | MW: ~6.5 kDa | Thrombin; trypsin; plasmin | [27] |
| Desmodusrotundus | Desmolaris | MW: 21.5 kDa | FXIa (KD=0.63 nmol/L); FXa; kallikrein | [28] |
| Eudiplozoon nipponicum | EnKT1 | MW: 10.12 kDa pI: 8.3 | FXa; trypsin; plasmin; PK | [31] |
| Delonixregia | AsTI | MW: 20 kDa | PK; FXa | [32] |
| Aschcia schweinfurthii var schweinfurthii | DrTI | PK; FXIIa; FXIa |
表选项
内源性Kunitz型丝氨酸蛋白酶抑制剂蛋白连接酶-2 (Protease nexin-2,PN2) 是淀粉样β蛋白前体(Amyloid β-protein precursor,AβPP) 的可溶形式,从活化的血小板中释放出来,包含一个Kunitz结构域,分子量为120 kDa[12]。PN2的Kunitz结构域(The Kunitz protease inhibitor domain of protease nexin-2,PN2KPI) 包含57个氨基酸(PN2序列中为Glu289-Ile345) 承担起PN2的全部FⅪa抑制功能[13]。PN2KPI通过Loop1 (11TGPCRAMISR20) 和Loop2 (34FYGGC38) 与FXIa催化结构域进行广泛的相互作用。PN2KPI中的Arg15(P1) 与FⅪa的Asp189相互作用形成S1口袋中的盐桥。P1′(Ala) 与P1在抑制剂与蛋白酶结合上起着重要作用[14]。Cys14(P2) 和Gly12(P4) 在Pro13(P3) 旋转骨架的帮助下与FⅪa的Lys192形成氢键,Met17(P2′) 和Ser19(P4′) 与FⅪa的Arg37D相互作用,高度保守的Phe(P18′) 通过内部疏水作用稳定反应位点的结构[15] (图 2A和2B)。静脉注射PN2KPI的野生型小鼠可显著减少FeCl3诱导的动脉血栓形成[12, 16]。另一种内源性Kunitz型丝氨酸蛋白酶抑制剂为组织因子途径抑制剂(Tissue factor pathway inhibitor,TFPI),它可通过阻断早期促凝刺激物而发挥抗凝活性[17]。TFPI在人体内主要有TFPIα和TFPIβ两个同工型,其中TFPIα有276个氨基酸残基,包括3个相连的Kunitz结构域(K1,Asp13-Arg78;K2,Glu92- Gly150;K3,Glu182-Lys241)[18]。K1和K2分别与活化凝血因子Ⅶ (Factor Ⅶa,FⅦa) 和FⅩa结合(图 2C),以抑制组织因子(Tissue factor,TF) 介导的凝血,而K3与辅因子蛋白S结合直接抑制FⅩa[19]。TFPI以一种依赖凝血因子Ⅷ(Factor Ⅷ,FⅧ) 和凝血因子Ⅸ (Factor Ⅸ,FⅨ) 存在的方式调节TF-FⅦa介导的FⅩa的产生。抑制TFPI可减轻血友病患者的出血情况,同时血小板TFPIα的选择性抑制剂已被证明可以调节血友病小鼠的出血,而治疗人类血友病的药物也已提上日程[20]。
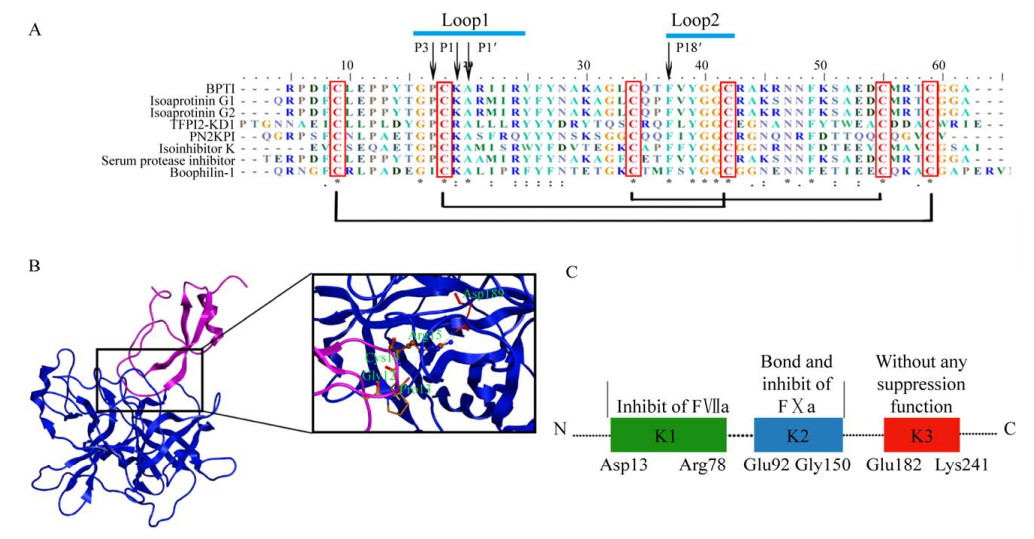 |
| 图 2 参与血凝平衡的Kunitz型丝氨酸蛋白酶抑制剂 Fig. 2 The Kunitz-type protease inhibitor involved in blood coagulation homeostasis. (A) Amino acid sequence alignment of different Kunitz inhibitors: BPTI, basic pancreatic trypsin inhibitor from Bos taurus (P00974); isoaprotinin G1 (Q7M311); isoaprotinin G2 (S10063); TFPI2-KD1, first Kunitz domain of tissue factor pathway inhibitor (P48307); PN2KPI, Kunitz domain of PN2 (1ZJD_B); isoinhibitor K (P00994); serum basic protease inhibitor (P00975) boophilin-1, first Kunitz domain of boophilin (CAC82583)[21]. P3, P1, P1′, P18′ are highlighted by black arrows, two loops are marked with short blue lines, and three pairs of disulfide bonds are connected by black lines. (B) Structure of the FⅪa catalytic domain in complex with the KPI domain of PN2 (PDB: 1ZJD). The ribbon structure shown in blue is the catalytic domain of FⅪa, whereas the ribbon structure shown in purple is the KPI domain of PN2, and key amino acids are shown in light green. (C) Primary structure of human TFPI. The three Kunitz domains are represented by green, blue and red respectively. K1: Asp13-Arg78; K2: Glu92-Gly150; K3: Glu182-Lys241. The K1 domain is responsible for inhibition of FⅦa, the K2 domain binds to and inhibits FⅩa, and K3 does not have any inhibitory function but appears to be involved in the binding of heparin-like substances. |
| 图选项 |
蛇毒是生物活性蛋白和多肽的丰富来源,毒素中Kunitz型丝氨酸蛋白酶抑制剂是研究最广泛的一类。Fasxiator是从带状金环蛇Bungarus fasciatus的毒液中分离并测序得到的Kunitz型丝氨酸蛋白酶抑制剂,其重组表达形式rFasxiator表现出与天然Fasxiator相似的二级结构以及对FⅪa和糜蛋白酶的抑制作用[21],是FⅪa缓慢有效的抑制剂。为了提高rFasxiator的效力和选择性,对rFasxiator的Loop1和Loop2中特定残基进行定点突变得到rFasxiatorN17R, L19E,该突变体在最高浓度为300 nmol/L时能2倍延长活化部分凝血酶原时间(Activated partial thromboplastin time,APTT),且在浓度为40 μmol/L时对人血浆凝血酶原时间(Prothrombin time,PT) 无显著影响[21-22]。在FeCl3诱导的血栓形成模型中,rFasxiatorN17R,L19E能够延长FeCl3诱导血栓形成模型中小鼠的颈动脉闭塞时间[21]。Kaur等[23]从金环蛇Bungarus flaviceps毒腺cDNA文库转录组中鉴定得到的Flavikunin也是一种Kunitz型丝氨酸蛋白酶抑制剂。野生型和突变型Flavikunin均能抑制纤溶酶和胰蛋白酶,但对糜蛋白酶、凝血酶、弹性蛋白酶等其他丝氨酸蛋白酶无明显抑制作用。Flavikunin的P1位组氨酸参与纤溶酶抑制,具有温和的抗凝血活性。在500 nmol/L条件下,Flavikunin对纤溶酶的抑制率约为57%。
吸血寄生虫向宿主体内释放多种抗凝血物质来阻止宿主血液凝固,因此许多与凝血相关的Kunitz型蛋白酶抑制剂如Ir-CPI[24]、Simukunin[25]、Ixolaris[26]、Boophilin[27]和Desmolaris[28]等均来源于食血寄生虫。Decrem等[24]从蜱虫Ixodes ricinus唾液腺cDNA文库中预测得到Ir-CPI与TFPI的第2个Kunitz结构域相似,它能够选择性抑制接触途径相关的凝血因子。Ir-CPI具有一个典型的Kunitz结构域,能与人血浆FⅪa、FⅫa和激肽释放酶特异性结合来抑制内在凝血途径,并轻微抑制体外纤维蛋白溶解。体外实验表明,Ir-CPI能显著延长APTT (2.0 μmol/L时为7.7倍) 而不改变PT[29]。同样来自黑蝇Simulium vittatum唾液腺的Simukunin也具有典型的Kunitz蛋白结构特征。Simukunin的作用可能类似于BPTI,P1位的Lys深入到胰蛋白酶的S1特异性口袋并与带负电荷的Asp189侧链形成极性相互作用[25]。重组Simukunin (rSimukunin) 显著抑制FⅩa和FⅪa,但不抑制凝血酶或FⅫa的活性。此外,rSimukunin还能强烈抑制弹性蛋白酶、纤溶酶、激肽释放酶、胰蛋白酶和组织蛋白酶G[30]。Jedli?ková等[31]从日本双身虫Eudiplozoon nipponicum转录组中鉴定出一种有效的分泌型抗凝血剂和补体抑制剂EnKT1,它与抗出血性蛇毒因子Textilinin-1在氨基酸序列上具有69%的相似度。EnKT1通过抑制FⅩa产生抗凝作用,但对纤溶作用无明显影响。此外,EnKT1还可能通过抑制FⅩa激活的C3和C5转化酶而削弱补体功能,这是破坏补体功能的寄生虫Kunitz抑制剂的第一个实例[31]。
此外,Salu等[32]从植物凤凰木Delonix regia和南非金合欢Acacia schweinfurthii中分离得到两种Kunitz型蛋白酶抑制剂DrTI和AsTI。它们通过干扰前激肽释放酶对内在凝血途径的蛋白发挥水解作用,从而导致血栓的改善。在体外实验中,DrTI (21 μmol/L) 和AsTI (15.4 μmol/L) 能2倍延长APTT且对PT没有显著影响,而在研究小鼠动脉血栓和出血时间模型中,DrTI和AsTI浓度分别为1.3 μmol/L和0.96 μmol/L时,小鼠颈动脉完全闭塞的时间较对照组延长了约50%。同时,相较于肝素,DrTI和AsTI引起的出血时间并未延长。
凝血级联蛋白功能失调与心脑血管疾病密切相关,而Kunitz型丝氨酸蛋白酶抑制剂展现出治疗血栓性疾病的巨大潜力。它们来源广泛,对其表征及结构相关鉴定也在陆续开展,但该类蛋白酶的结构与功能关系还有待于深入研究。
2.2 抗感染功能抗生素的发现彻底改变了对感染的控制。然而,耐药性的产生同样限制着抗生素的发展。寻找具有新型作用机制的抗菌剂控制细菌和真菌感染越来越引起人们的重视。Kunitz型丝氨酸蛋白酶抑制剂在对抗各种感染中表现出卓越优势。目前,人们已从多种动植物中成功分离出Kunitz型丝氨酸蛋白酶抑制剂,并对其生物学功能进行了深入研究。
存在于植物种子和块茎中的蛋白酶抑制剂在植物抵御昆虫、真菌和其他病原细菌攻击的防御系统中发挥着重要作用。目前已从植物中分离出许多具有抗菌活性的蛋白酶抑制剂,并对其进行了详细的鉴定。Cai等[33]从药用植物太子参新鲜根中分离出Kunitz型抑制剂PHTI。抑制动力学研究表明,PHTI是一种竞争性抑制剂,具有抗真菌的作用,可通过破坏细胞膜而抑制炭疽菌和尖孢镰刀菌。
De Oliveira等[34]从青皮象耳豆Enterolobium timbouva种子中分离得到一种双功能Kunitz蛋白酶抑制剂EtTI。与典型的Kunitz结构域不同,EtTI分子中有4个半胱氨酸残基形成两对链间二硫键来连接异源二聚体。EtTI对白色念珠菌、布氏念珠菌和热带念珠菌均有抗菌活性,可能通过细胞凋亡引起质膜完整性紊乱和形态改变(表 2)。Dib等[35]首次从巴西水葫芦中纯化出一种胰蛋白酶抑制剂IETI。它通过改变酵母细胞膜通透性来表现其抗真菌活性,具有作为抗真菌药物的潜在用途。
表 2 参与抗感染过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质Table 2 Origins and properties of Kunitz-type serine protease inhibitors involved in the anti-infection process
| Organism | Peptide/protein | Physical and chemical properties | Microorganisms inhibited | Enzymes inhibited | References |
| Pseudostellaria heterophylla | PHTI | MW: 20.5 kDa | Phytopathogens; Colletotrichum gloeosporioides; Fusarium oxysporum | Trypsin (Ki=3.01×10?9 mol/L) | [33] |
| Enterolobium timbouva seeds | EtTI | MW: 19 kDa | C. albicans, C. buinensis; C. tropicalis | Trypsin (Ki=0.5 nmol/L) | [34] |
| Inga edulis | IETI | MW: 19 kDa | C. buinensis; C. tropicalis | Trypsin (Ki=6.2 nmol/L) | [35] |
| Paralichthys olivaceus | PoKspi | MW: 56.5 kDa pI: 5.44 | Gram-negative; Gram-positive | Trypsin | [36] |
| Hyriopsis cumingii | HcKuPI | MW: 9.5 kDa pI: 9.96 | Gram-positive; Gram-negative | Trypsin | [37] |
| Silkworm cocoon | BmSPI51 | MW: 6.03 kDa | Candida albicans; Beauveria bassiana; Saccharomyces cerevisiae | – | [39] |
表选项
已有越来越多的研究证明海洋动物体内存在的Kunitz型丝氨酸蛋白酶抑制剂具有免疫防御能力。病毒或细菌感染会显著上调对虾、扇贝、梭子鱼和鲫鱼中该类型抑制剂mRNA的表达[36]。Xu等[36]从日本比目鱼中鉴定出一种含有2个Kunitz结构域的丝氨酸蛋白酶PoKspi。重组表达的PoKspi能够同时抑制革兰氏阴性菌和革兰氏阳性菌。Pokspi还是一种调理素,可下调肿瘤坏死因子-α (Tumor necrosis factor-α,TNFα) 和IL-1β的表达,保护组织免受促炎因子引起过度的炎症反应。蛋白酶抑制剂是软体动物贝壳有机基质中的常见成分,通常与免疫相关(表 2)。Jin等[37]从三角帆蚌中分离到一种Kunitz丝氨酸蛋白酶抑制剂HcKuPI,该分子能在珍珠形成过程中发挥抗菌作用,这些发现扩展了我们对蛋白酶抑制剂在免疫系统和贝壳生物矿化中的作用的认识。
在蚕茧中已经鉴定出一系列具有不同功能的Kunitz蛋白酶抑制剂[38],其中BmSPI51含量最高,占蚕茧蛋白酶抑制剂的67%。体外抑制试验表明,BmSPI51对白色念珠菌、球孢白僵菌以及酿酒酵母菌3种真菌的孢子生长均有明显的抑制作用。BmSPI51通过与细胞壁多糖、甘露聚糖和β-葡聚糖结合来抑制真菌生长[39]。参与抗感染过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质详见表 2。
与一般抗生素不同,Kunitz型丝氨酸蛋白酶抑制剂具有广谱抗菌性、不易引起耐药性等优势,已经得到广泛的关注,有望被开发成新一代肽类抗生素。但是其生产成本较高、溶血毒副作用强、治疗指数低等缺点,成为目前临床上应用的主要障碍,限制了其进一步发展。为了改善这一劣势,科研人员对其结构进行改造并已取得相应理论依据,在不久的将来,Kunitz型丝氨酸蛋白酶抑制剂将会成为临床上解决耐药问题的重要生物制剂。
2.3 炎症调节作用蠕虫寄生虫中存在的Kunitz型丝氨酸蛋白酶抑制剂与对炎症反应的保护有关[40]。目前已在人体多种组织粘膜中发现内源性丝氨酸蛋白酶抑制剂Bikunin。Bikunin能通过抑制脂多糖(Lipopolysaccharide,LPS) 诱导的肿瘤坏死因子发挥抗炎作用,是治疗炎症和感染性休克的候选药物[41]。Falcón等[42]从肝片形吸虫Fasciola hepatica的肠道和外皮分离得到Kunitz型丝氨酸蛋白酶抑制剂Fh-KTM,该分子能够与宿主免疫防御系统密切接触并通过下调LPS诱导的树突状细胞成熟来参与炎症调节和免疫反应。Ranasinghe等[43]从曼氏血吸虫成虫和卵的排泄物中分离得到一种Kunitz型蛋白酶抑制剂rSmKI-1,该分子与TFPI-2具有57%氨基酸序列相似性[44]。rSmKI-1免疫的小鼠分泌干扰素γ,白介素(Interleukin,IL)-10和IL-6增加,在保护寄生虫免受宿主防御攻击方面起着特殊作用[43-44]。此外,在日本血吸虫的热休克蛋白60中分离到一种名为SJMHE1的小分子多肽,它同样具有抗凝和抗炎的特性。SJMHE1可抑制过敏性小鼠的气道炎症,减少肺和支气管肺泡灌洗液中炎症细胞的浸润,调节过敏性小鼠脾细胞和肺组织中促炎因子的产生,抑制小鼠迟发型超敏反应和胶原诱导性关节炎,在治疗哮喘和其他过敏性或炎症性疾病药物开发过程中提供新思路[45]。参与炎症调节过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质详见表 3。
表 3 参与炎症调节过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质Table 3 Origins and properties of Kunitz-type serine protease inhibitors involved in the process of inflammation regulation
| Organism | Peptide/protein | MW | Enzymes inhibited | Activity | References |
| Fasciola hepatica | Fh-KTM | ~10 kDa | Neutrophil elastase | Hydrolase | [42] |
| Schistosoma mansoni | SmKI-1 | 16 kDa | Neutrophil elasta (Ki=56 nmol/L); PK (Ki=112 nmol/L) | Trypsin | [43-44] |
| Schistosoma japonicum | SJMHE1 | – | – | Trypsin | [45] |
| Boophilus microplus | rBmTI-6 | ~8 kDa | Trypsin (Ki=1.7 nmol/L) | – | [46] |
| Human | Bikunin | 43 kDa | Neutrophil elastase | – | [41] |
表选项
Kunitz丝氨酸蛋白酶抑制剂是炎症病理学研究的主要贡献者,作为治疗炎症性疾病药物已经引起了人们的浓厚兴趣。特别是来自曼氏血吸虫的SmKI-1已被证明能够干扰中性粒细胞弹性蛋白酶的活性,在不同炎症性疾病模型中减少炎症发生,可能是未来作为疫苗或药物靶标进行评估的良好候选物。
2.4 抗肿瘤转移和迁徙内皮细胞迁移和血管生成导致肿瘤转移。近年来,有效的蛋白酶抑制剂作为潜在的抗癌药物得到了广泛的研究。
内源性丝氨酸蛋白酶抑制剂的单一Kunitz结构域如肝细胞生长因子激活抑制剂1型(Hepatocyte growth factor activator inhibitor-1,HAI-1)[47]和TFPI-2[48]即可表现出良好的抗肿瘤转移和迁徙作用。
Ranasinghe等[49]从犬绦虫中鉴定出的一种有效的Kunitz型丝氨酸蛋白酶抑制剂EgkI-1。该分子能显著抑制小鼠模型中黑色素瘤的生长,并且对周围正常组织没有毒性,在体内显示出有效的抗癌活性。此外,EgKI-1能显著降低腋窝淋巴结引流部位生存素的表达水平,增加了CD8+ T细胞的数量。EgKI-1不仅可以作为黑色素瘤的局部治疗药物,还能以直接或间接诱导肿瘤细胞凋亡的方式控制肿瘤迁徙和转移,是一个很有开发前景的多肽分子,进一步评估EgKI-1抗肿瘤潜力并确定其杀死癌细胞的机制可能对未来抗肿瘤药物的开发提供新的思路。Amblyomin-X是成年蜱虫唾液腺转录组中鉴定得到的丝氨酸蛋白酶抑制剂[50],体内实验表明,它可以通过调节尿激酶型纤溶酶原激活剂受体信号减少基质金属蛋白酶(Matrix metalloproteinase,MMP) 的释放,特异性地针对肿瘤细胞转移而对正常细胞影响较小。Amblyomin-X还可以使Bcl-2家族蛋白失衡诱导癌细胞凋亡。在黑色素瘤动物模型中Amblyomin-X选择性诱导肿瘤细胞凋亡,促进体内肿瘤缩小,在小鼠原位肾肿瘤模型中显著减少肺转移[51]。目前Amblyomin-X作为一种新的潜在的抗肿瘤药物已进行临床前研究[52]。
许多研究表明,植物Kunitz型丝氨酸蛋白酶抑制剂可能是一种很有前途的新型抗癌药物的来源。Yang等[53]从甘薯中分离出Kunitz型丝氨酸蛋白酶抑制剂Sporamin,可减弱肝细胞内蛋白质的合成以及炎性细胞向肝脏的浸润,在小鼠模型中,Sporamin显著减少了腹腔中形成的肿瘤结节的数量和重量[53]。Fang等[54]首次从紫荆花种子中发现一种新的Kunitz型胰蛋白酶抑制剂并命名为BvvTI,该分子具有显著的抗HIV-1逆转录酶活性和抗鼻咽癌CNE-1活性。参与肿瘤免疫过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质详见表 4。
表 4 参与肿瘤免疫过程的Kunitz型丝氨酸蛋白酶抑制剂的来源和性质Table 4 Origins and properties of Kunitz-type serine protease inhibitors involved in tumor immune process
| Organism | Peptide/protein | MW | Tumor model | Enzymes inhibited | References |
| Echinococcusgranulosus | EgkI-1 | – | Breast cancer; melanoma | Neutrophil elastase | [49] |
| Amblyommacajennense | Amblyomin-X | 15 kDa | Melanoma; pediatric anaplastic ependymoma | Matrix metallo proteinases | [50-52] |
| Ipomoeabatatas | Sporamin | – | Colorectal cancer; tongue carcinoma; pancreatic cancer | Liver β-catenin; vascular endothelial growth factor | [53] |
| Bauhinia variegata var. variegata | BvvTI | 21 kDa | Anti-HIV-1 reverse transcriptase | Trypsin (Ki=0.1×10?9 mol/L); Chymotrypsin (Ki=250.5×10?9 mol/L) | [54] |
| Flowcytometry | APPI-4M | – | Colon, gastric; lung; ovarian cancers | Kallikrein | [55] |
表选项
通过探索更多新颖的Kunitz抑制剂,并研究它们与肿瘤细胞的相互作用关系,对抗癌药物的发现具有深远的影响。
2.5 其他功能除上述功能,De Medeiros等[56]还从罗望子种子中分离纯化得到胰蛋白酶抑制剂pTTI。pTTI表现出抗TNFα活性,能够降低大鼠血脂异常和肥胖引起的脂蛋白水平提高[57]。Liao等[58]利用大规模测序和数据分析相结合的策略,结合转录组学和蛋白质组学鉴定得到Kunitz型多肽ZoaKuz1,该分子可以减缓甚至阻止离子通道过度活动介导的神经退行性病变来缓解神经功能障碍。Smith等[59]还通过分离纯化手段得到一种58 kDa的绵羊关节软骨丝氨酸蛋白酶抑制剂,可以保护软骨表面成分不被蛋白质降解,保持关节功能。
3 Kunitz型丝氨酸蛋白酶抑制剂药物的开发研究从天然动植物中获取的先导分子一直是药物开发和疾病治疗的重要来源。Kunitz结构域在蛋白质工程领域获得了极大关注,利用噬菌体展示技术发现了许多含Kunitz结构域的多肽,其中有些多肽已被美国食品药品监督管理局(Food and Drug Administration,FDA) 批准上市。这些药物中第一个上市的是激肽释放酶抑制剂Ecallantide[60],该药于2009年获批,商品名为Kalbitor,用于皮下注射,适用于治疗16岁以上患者遗传性血管水肿急性发作[61]。Ecallantide通过噬菌体展示技术发现并由毕赤酵母重组表达,由60个氨基酸组成,含有3对分子内二硫键,与人TFPI的氨基酸序列相差7个氨基酸,能参与激肽释放酶-激肽系统,选择性且高效可逆地抑制人血浆激肽释放酶,从而抑制高分子激肽原产生缓激肽。1992年,Dunlevy等同样利用噬菌体展示技术从BPTI突变体文库中筛选特异性结合弹性蛋白酶的Kunitz多肽,产生的工程化分子Depelestat[62]能够抑制囊性纤维化患者痰中人嗜中性粒细胞弹性蛋白酶的活性,在2007年,该药已通过Ⅱ期临床试验,用于治疗急性呼吸窘迫综合征[63]。此外,从胰腺中提取的BPTI对胰蛋白酶、糜蛋白酶、溶血纤维蛋白酶及各种组织或血浆激肽释放酶有较好的抑制能力,因此被用于减少心脏和肝脏手术等复杂手术中的出血[64]。
4 总结与展望Kunitz型丝氨酸蛋白酶抑制剂来源广泛,种类丰富,主要获取方式有以下几种:1) 天然产物分离纯化;2) 转录组学和蛋白质组学鉴定;3) 噬菌体表面展示技术筛选等。目前,通过结构功能预测及生物学验证,已获得上百种具有特定功能的Kunitz型丝氨酸蛋白酶抑制剂。其分子量小,具有广泛的温度和pH稳定性,不易被各种酶降解,半衰期较长,其中抗炎、抗癌、调节蛋白酶和细胞因子等作用正在不断地应用于临床,引起了人们对其作为疾病治疗药物的浓厚兴趣。但是分析目前鉴定得到的Kunitz型丝氨酸蛋白酶抑制剂,也存在一些问题:1) 专属性不够理想。比如具有调节血凝平衡的PN2KPI不仅能抑制FⅪa还能以更强的能力抑制胰蛋白酶,作为抗栓药物可能会带来脱靶效应和副作用。2) 抗感染机制研究不够充分。多数具有抗感染作用的Kunitz型丝氨酸蛋白酶抑制剂,对其各种真菌和细菌的抑制能力进行了充分的探索和验证,但是具体抑制机制尚不明确。3) Kunitz型丝氨酸蛋白酶抑制剂多采用注射给药,这可能会影响患者的用药顺应性。4) Kunitz多肽在免疫调节、抗炎和抗癌方面仅有初步的研究,其作用机制及临床应用还有待进一步研究。
笔者所在研究团队充分结合转录组、蛋白质组和多肽组从五步蛇、银环蛇和水蛭中共鉴定得到33条具有抑制FⅪa能力的Kunitz型丝氨酸蛋白酶抑制剂。其中从五步蛇毒腺转录组数据库中挖掘得到的Kunitz型多肽对FⅪa、胰蛋白酶和激肽释放酶具有较好的抑制作用,从水蛭中鉴定到的Kunitz型多肽也被成功克隆表达及活性分析,这些多肽同样具有显著的FⅪa抑制活性。相信随着认识的不断深入,Kunitz型丝氨酸蛋白酶抑制剂在医药领域将会有更加广泛的应用,为人类健康带来更多的福音。
参考文献
| [1] | Boon L, Ugarte-Berzal E, Vandooren J, et al. Protease propeptide structures, mechanisms of activation, and functions. Crit Rev Biochem Mol Biol, 2020, 55(2): 111-165. DOI:10.1080/10409238.2020.1742090 |
| [2] | Leung D, Abbenante G, Fairlie DP. Protease inhibitors: current status and future prospects. J Med Chem, 2000, 43(3): 305-341. DOI:10.1021/jm990412m |
| [3] | Harish BS, Uppuluri KB. Microbial serine protease inhibitors and their therapeutic applications. Int J Biol Macromol, 2018, 107: 1373-1387. DOI:10.1016/j.ijbiomac.2017.09.115 |
| [4] | Ranasinghe S, McManus DP. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev Comp Immunol, 2013, 39(3): 219-227. DOI:10.1016/j.dci.2012.10.005 |
| [5] | Wei XM, Yang JL, Yang JM, et al. A four-domain Kunitz-type proteinase inhibitor from Solen grandis is implicated in immune response. Fish Shellfish Immunol, 2012, 33(6): 1276-1284. DOI:10.1016/j.fsi.2012.09.015 |
| [6] | Kunitz M, Northrop JH. Isolation from beef pancreas of crystalline trypsinogen, trypsin, a trypsin inhibitor, and an inhibitor-trypsin compound. J Gen Physiol, 1936, 19(6): 991-1007. DOI:10.1085/jgp.19.6.991 |
| [7] | Mishra M. Evolutionary aspects of the structural convergence and functional diversification of Kunitz-domain inhibitors. J Mol Evolut, 2020, 88(7): 537-548. DOI:10.1007/s00239-020-09959-9 |
| [8] | Bendre AD, Ramasamy S, Suresh CG. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int J Biol Macromol, 2018, 113: 933-943. DOI:10.1016/j.ijbiomac.2018.02.148 |
| [9] | Moiola M, Memeo MG, Quadrelli P. Stapled peptides—a useful improvement for peptide-based drugs. Molecules, 2019, 24(20): 3654. DOI:10.3390/molecules24203654 |
| [10] | Cohen I, Coban M, Shahar A, et al. Disulfide engineering of human Kunitz-type serine protease inhibitors enhances proteolytic stability and target affinity toward mesotrypsin. J Biol Chem, 2019, 294(13): 5105-5120. DOI:10.1074/jbc.RA118.007292 |
| [11] | Grzesiak A, Helland R, Smal?s AO, et al. Substitutions at the P1' position in BPTI strongly affect the association energy with serine proteinases. J Mol Biol, 2000, 301(1): 205-217. DOI:10.1006/jmbi.2000.3935 |
| [12] | Sheffield WP, Eltringham-Smith LJ, Bhakta V. Fusion to human serum albumin extends the circulatory half-life and duration of antithrombotic action of the Kunitz protease inhibitor domain of protease nexin 2. Cell Physiol Biochem, 2018, 45(2): 772-782. DOI:10.1159/000487168 |
| [13] | Navaneetham D, Sinha D, Walsh PN. Walsh. Mechanisms and specificity of factor Ⅺa and trypsin inhibition by protease nexin 2 and basic pancreatic trypsin inhibitor. J Biochem, 2010, 148(4): 467-479. DOI:10.1093/jb/mvq080 |
| [14] | Navaneetham D, Wu WM, Li HB, et al. P1 and P2' site mutations convert protease nexin-2 from a factor Ⅺa inhibitor to a plasmin inhibitor. J Biochem, 2013, 153(2): 221-231. DOI:10.1093/jb/mvs133 |
| [15] | Su YC, Miller TN, Navaneetham D, et al. The role of factor Ⅺa (FⅪa) catalytic domain exosite residues in substrate catalysis and inhibition by the Kunitz protease inhibitor domain of protease nexin 2. J Biol Chem, 2011, 286(36): 31904-31914. DOI:10.1074/jbc.M111.257527 |
| [16] | Wu WM, Li HB, Navaneetham D, et al. The Kunitz protease inhibitor domain of protease nexin-2 inhibits factor Ⅺa and murine carotid artery and middle cerebral artery thrombosis. Blood, 2012, 120(3): 671-677. DOI:10.1182/blood-2012-03-419523 |
| [17] | Vadivel K, Ponnuraj SM, Kumar Y, et al. Platelets contain tissue factor pathway inhibitor-2 derived from megakaryocytes and inhibits fibrinolysis. J Biol Chem, 2014, 289(45): 31647-31661. DOI:10.1074/jbc.M114.569665 |
| [18] | Petersen LC. Hemostatic properties of a TFPI antibody. Thromb Res, 2012, 129(Suppl 2): S44-S45. |
| [19] | Chowdary P. Anti-tissue factor pathway inhibitor (TFPI) therapy: a novel approach to the treatment of haemophilia. Int J Hematol, 2020, 111(1): 42-50. DOI:10.1007/s12185-018-2548-6 |
| [20] | Peterson JA, Maroney SA, Mast AE. Mast. Targeting TFPI for hemophilia treatment. Thromb Res, 2016, 14(Suppl 2): S28-S30. |
| [21] | Chen W, Carvalho LP, Chan MY, et al. Fasxiator, a novel factor Ⅺa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J Thromb Haemost, 2015, 13(2): 248-261. DOI:10.1111/jth.12797 |
| [22] | Van Der Beelen SHE, Agten SM, Suylen DPL, et al. Design and synthesis of a multivalent catch-and-release assay to measure circulating FⅪa. Thromb Res, 2021, 200: 16-22. DOI:10.1016/j.thromres.2021.01.002 |
| [23] | Kaur S, Devi A, Saikia B, et al. Expression and characterization of Flavikunin: a Kunitz-type serine protease inhibitor identified in the venom gland cDNA library of Bungarus flaviceps. J Biochem Mol Toxicol, 2019, 33(4): e22273. DOI:10.1002/jbt.22273 |
| [24] | Decrem Y, Rath G, Blasioli V, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med, 2009, 206(11): 2381-2395. DOI:10.1084/jem.20091007 |
| [25] | Al-Horani RA, Desai UR. Factor Ⅺa inhibitors: a review of the patent literature. Exp Opin Ther Pat, 2016, 26(3): 323-345. DOI:10.1517/13543776.2016.1154045 |
| [26] | De Paula VS, Sgourakis NG, Francischetti IMB, et al. NMR structure determination of ixolaris and factor Ⅹ(a) interaction reveals a noncanonical mechanism of Kunitz inhibition. Blood, 2019, 134(8): 699-708. DOI:10.1182/blood.2018889493 |
| [27] | Macedo-Ribeiro S, Almeida C, Calisto BM, et al. Isolation, cloning and structural characterisation of boophilin, a multifunctional Kunitz-type proteinase inhibitor from the cattle tick. PLoS ONE, 2008, 3(2): e1624. DOI:10.1371/journal.pone.0001624 |
| [28] | Ma DY, Mizurini DM, Assump??o TCF, et al. Desmolaris, a novel factor Ⅺa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood, 2013, 122(25): 4094-4106. DOI:10.1182/blood-2013-08-517474 |
| [29] | Pireaux V, Tassignon J, Demoulin S, et al. Anticoagulation with an inhibitor of factors Ⅺa and Ⅻa during cardiopulmonary bypass. J Am Coll Cardiol, 2019, 74(17): 2178-2189. DOI:10.1016/j.jacc.2019.08.1028 |
| [30] | Al-Horani RA, Afosah DK. Recent advances in the discovery and development of factor Ⅺ/Ⅺa inhibitors. Med Res Rev, 2018, 38(6): 1974-2023. DOI:10.1002/med.21503 |
| [31] | Jedli?ková L, Dvo?ák J, Hrachovinová I, et al. A novel Kunitz protein with proposed dual function from Eudiplozoon nipponicum (Monogenea) impairs haemostasis and action of complement in vitro. Int J Parasitol, 2019, 49(5): 337-346. DOI:10.1016/j.ijpara.2018.11.010 |
| [32] | Salu BR, Pando SC, Brito MV, et al. Improving the understanding of plasma kallikrein contribution to arterial thrombus formation using two plant protease inhibitors. Platelets, 2019, 30(3): 305-313. DOI:10.1080/09537104.2018.1428738 |
| [33] | Cai XX, Xie XL, Fu NY, et al. Physico-chemical and antifungal properties of a trypsin inhibitor from the roots of Pseudostellaria heterophylla. Molecules, 2018, 23(9): 2388. DOI:10.3390/molecules23092388 |
| [34] | De Oliveira CFR, Oliveira CT, Taveira GB, et al. Characterization of a Kunitz trypsin inhibitor from Enterolobium timbouva with activity against Candida species. Int J Biol Macromol, 2018, 119: 645-653. DOI:10.1016/j.ijbiomac.2018.07.034 |
| [35] | Dib HX, De Oliveira DGL, De Oliveira CFR, et al. Biochemical characterization of a Kunitz inhibitor from Inga edulis seeds with antifungal activity against Candida spp. Arch Microbiol, 2019, 201(2): 223-233. DOI:10.1007/s00203-018-1598-8 |
| [36] | Xu X, Liu JX, Wang YJ, et al. Kunitz-type serine protease inhibitor is a novel participator in anti-bacterial and anti-inflammatory responses in Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol, 2018, 80: 22-30. DOI:10.1016/j.fsi.2018.05.058 |
| [37] | Jin C, Liu XJ, Li JL. A Kunitz proteinase inhibitor (HcKuPI) participated in antimicrobial process during pearl sac formation and induced the overgrowth of calcium carbonate in Hyriopsis cumingii. Fish Shellfish Immunol, 2019, 89: 437-447. DOI:10.1016/j.fsi.2019.04.021 |
| [38] | Li YS, Liu HW, Zhu R, et al. Protease inhibitors in Bombyx mori silk might participate in protecting the pupating larva from microbial infection. Insect Sci, 2016, 23(6): 835-842. DOI:10.1111/1744-7917.12241 |
| [39] | Zhang XL, Guo KY, Dong ZM, et al. Kunitz-type protease inhibitor BmSPI51 plays an antifungal role in the silkworm cocoon. Insect Biochem Mol Biol, 2020, 116: 103258. DOI:10.1016/j.ibmb.2019.103258 |
| [40] | De Magalh?es MTQ, Mambelli FS, Santos BPO, et al. Serine protease inhibitors containing a Kunitz domain: their role in modulation of host inflammatory responses and parasite survival. Microbes Infect, 2018, 20(9/10): 606-609. |
| [41] | Lepedda AJ, De Muro P, Capobianco G, et al. Role of the small proteoglycan bikunin in human reproduction. Hormones (Athens), 2020, 19(2): 123-133. DOI:10.1007/s42000-019-00149-x |
| [42] | Falcón CR, Masih D, Gatti G, et al. Fasciola hepatica Kunitz type molecule decreases dendritic cell activation and their ability to induce inflammatory responses. PLoS ONE, 2014, 9(12): e114505. DOI:10.1371/journal.pone.0114505 |
| [43] | Ranasinghe SL, Fischer K, Gobert GN, et al. Functional expression of a novel Kunitz type protease inhibitor from the human blood fluke Schistosoma mansoni. Parasit Vectors, 2015, 8: 408. DOI:10.1186/s13071-015-1022-z |
| [44] | Ranasinghe SL, Duke M, Harvie M, et al. Kunitz-type protease inhibitor as a vaccine candidate against schistosomiasis mansoni. Int J Infect Dis, 2018, 66: 26-32. DOI:10.1016/j.ijid.2017.10.024 |
| [45] | Zhang WZ, Li L, Zheng Y, et al. Schistosoma japonicum peptide SJMHE1 suppresses airway inflammation of allergic asthma in mice. J Cell Mol Med, 2019, 23(11): 7819-7829. DOI:10.1111/jcmm.14661 |
| [46] | Duran AFA, Neves LDP, Da Silva FRS, et al. rBmTI-6 attenuates pathophysiological and inflammatory parameters of induced emphysema in mice. Int J Biol Macromol, 2018, 111: 1214-1221. DOI:10.1016/j.ijbiomac.2018.01.066 |
| [47] | Hong ZB, De Meulemeester L, Jacobi A, et al. Crystal structure of a two-domain fragment of hepatocyte growth factor activator inhibitor-1: functional interactions between the Kunitz-type inhibitor domain-1 and the neighboring polycystic kidney disease-like domain. J Biol Chem, 2016, 291(27): 14340-14355. DOI:10.1074/jbc.M115.707240 |
| [48] | Xu CD, Deng F, Mao ZH, et al. The interaction of the second Kunitz-type domain (KD2) of TFPI-2 with a novel interaction partner, prosaposin, mediates the inhibition of the invasion and migration of human fibrosarcoma cells. Biochem J, 2012, 441(2): 665-674. DOI:10.1042/BJ20110533 |
| [49] | Ranasinghe SL, Fischer K, Zhang WB, et al. Cloning and characterization of two potent kunitz type protease inhibitors from Echinococcus granulosus. PLoS Negl Trop Dis, 2015, 9(12): e0004268. DOI:10.1371/journal.pntd.0004268 |
| [50] | Schmidt MCB, Morais KLP, De Almeida MES, et al. Amblyomin-X, a recombinant Kunitz-type inhibitor, regulates cell adhesion and migration of human tumor cells. Cell Adh Migr, 2020, 14(1): 129-138. DOI:10.1080/19336918.2018.1516982 |
| [51] | Boufleur P, Sciani JM, Goldfeder M, et al. Biodistribution and pharmacokinetics of amblyomin-Ⅹ, a novel antitumour protein drug in healthy mice. Eur J Drug Metab Pharmacokinet, 2019, 44(1): 111-120. DOI:10.1007/s13318-018-0500-z |
| [52] | Maria DA, Will SEAL, Bosch RV, et al. Preclinical evaluation of amblyomin-Ⅹ, a Kunitz-type protease inhibitor with antitumor activity. Toxicol Rep, 2019, 6: 51-63. DOI:10.1016/j.toxrep.2018.11.014 |
| [53] | Yang C, Zhang JJ, Zhang XP, et al. Sporamin suppresses growth of xenografted colorectal carcinoma in athymic BALB/c mice by inhibiting liver β-catenin and vascular endothelial growth factor expression. World J Gastroenterol, 2019, 25(25): 3196-3206. DOI:10.3748/wjg.v25.i25.3196 |
| [54] | Fang EF, Wong JH, Bah CSF, et al. Bauhinia variegata var. variegata trypsin inhibitor: from isolation to potential medicinal applications. Biochem Biophys Res Commun, 2010, 396(4): 806-811. DOI:10.1016/j.bbrc.2010.04.140 |
| [55] | Sananes A, Cohen I, Shahar A, et al. A potent, proteolysis-resistant inhibitor of kallikrein-related peptidase 6 (KLK6) for cancer therapy, developed by combinatorial engineering. J Biol Chem, 2018, 293(33): 12663-12680. DOI:10.1074/jbc.RA117.000871 |
| [56] | De Medeiros AF, Costa IDS, De Carvalho FMC, et al. Biochemical characterisation of a Kunitz-type inhibitor from Tamarindus indica L. seeds and its efficacy in reducing plasma leptin in an experimental model of obesity. J Enzyme Inhib Med Chem, 2018, 33(1): 334-348. DOI:10.1080/14756366.2017.1419220 |
| [57] | Ribeiro JADNC, Serquiz AC, Silva PFDS, et al. Trypsin inhibitor from tamarindus indica L. seeds reduces weight gain and food consumption and increases plasmatic cholecystokinin levels. Clinics (Sao Paulo), 2015, 70(2): 136-143. DOI:10.6061/clinics/2015(02)11 |
| [58] | Liao QW, Gong GY, Poon TCW, et al. Combined transcriptomic and proteomic analysis reveals a diversity of venom-related and toxin-like peptides expressed in the mat anemone Zoanthus natalensis (Cnidaria, Hexacorallia). Arch Toxicol, 2019, 93(6): 1745-1767. DOI:10.1007/s00204-019-02456-z |
| [59] | Smith SM, Melrose J. A retrospective analysis of the cartilage Kunitz protease inhibitory proteins identifies these as members of the inter-α-trypsin inhibitor superfamily with potential roles in the protection of the articulatory surface. Int J Mol Sci, 2019, 20(3): 497. DOI:10.3390/ijms20030497 |
| [60] | Lehmann A. Ecallantide (DX-88), a plasma kallikrein inhibitor for the treatment of hereditary angioedema and the prevention of blood loss in on-pump cardiothoracic surgery. Expert Opin Biol Ther, 2008, 8(8): 1187-1199. DOI:10.1517/14712598.8.8.1187 |
| [61] | Zuraw B, Yasothan U, Kirkpatrick P. Ecallantide. Nat Rev Drug Discov, 2010, 9(3): 189-190. DOI:10.1038/nrd3125 |
| [62] | Dunlevy FK, Martin SL, De Courcey F, et al. Anti-inflammatory effects of DX-890, a human neutrophil elastase inhibitor. J Cyst Fibros, 2012, 11(4): 300-304. DOI:10.1016/j.jcf.2012.02.003 |
| [63] | Attucci S, Gauthier A, Korkmaz B, et al. EPI-hNE4, a proteolysis-resistant inhibitor of human neutrophil elastase and potential anti-inflammatory drug for treating cystic fibrosis. J Pharmacol Exp Ther, 2006, 318(2): 803-809. DOI:10.1124/jpet.106.103440 |
| [64] | Mahdy AM, Webster NR. Perioperative systemic haemostatic agents. Br J Anaesth, 2004, 93(6): 842-858. DOI:10.1093/bja/aeh227 |
