
 , 姚晓含, 马胜男, 张利静, 秦志海
, 姚晓含, 马胜男, 张利静, 秦志海

郑州大学 第一附属医院 医学研究中心 内分泌与代谢病科,河南 郑州 450052
收稿日期:2020-03-30;接收日期:2020-07-20;网络出版时间:2020-08-13
基金项目:国家自然科学基金(Nos. 31670881, 81630068),河南省重点研发与推广专项(科技攻关) (No. 202102310490),中国博士后科学基金(No. 2019M662538)资助
摘要:转移是肿瘤患者死亡最常见的原因,而淋巴转移是大多数肿瘤转移的主要途径之一。近年来,CC趋化因子配体21 (CC chemokine ligand 21,CCL21)及其受体CC趋化因子受体7型(CC chemokine receptor type 7,CCR7)在淋巴转移中的作用逐渐受到关注。CCL21主要由淋巴内皮细胞产生,其与树突状细胞(Dendritic cells,DCs)和T细胞等表面CCR7的相互作用是免疫细胞淋巴迁移及淋巴结归巢的主要决定因素。然而,表达CCR7的肿瘤细胞也可以利用类似的机制进入淋巴管进行淋巴转移。如何靶向CCL21/CCR7轴,既能抑制淋巴转移,又不影响抗肿瘤免疫反应已成为肿瘤免疫治疗研究的重要议题。文中将对CCL21/CCR7轴在淋巴转移中的作用及其作为靶点治疗肿瘤转移的临床前和临床试验研究进行综述,为靶向CCL21/CCR7信号轴治疗肿瘤转移的相关研究提供参考。
关键词:肿瘤淋巴转移CC趋化因子配体21CC趋化因子受体7型靶向治疗
Progress in targeting therapy of cancer metastasis by CCL21/CCR7 axis
Li Zhang, Fazhan Wang

 , Xiaohan Yao, Shengnan Ma, Lijing Zhang, Zhihai Qin
, Xiaohan Yao, Shengnan Ma, Lijing Zhang, Zhihai Qin

Medical Research Center, Department of Endocrinology and Metabolism, the First Affiliated Hospital of Zhengzhou University, Zhengzhou University, Zhengzhou 450052, Henan, China
Received: March 30, 2020; Accepted: July 20, 2020; Published: August 13, 2020
Supported by: National Natural Science Foundation of China (Nos. 31670881, 81630068), Key Research and Development Project of Henan Province (No. 202102310490), China Postdoctoral Science Foundation (No. 2019M662538)
Corresponding author: Fazhan Wang. E-mail: Fazhanwang_16@163.com;
Zhihai Qin. Tel: +86-371-66913620; E-mail: zhihai@ibp.ac.cn.
Abstract: Metastasis is the leading cause of mortality for cancer patients, and lymphatic metastasis is one of the main ways of tumor metastasis. The role of CCL21 and its receptor CCR7 in lymphatic metastasis has been increasingly concerned in recent years. CCR7 is mainly expressed by both dendritic cells and T cells for immune responses. CCL21, the chemokine ligand for CCR7, secreted from lymphatic endothelial cells binds CCR7 and recruits immune cells toward lymphatic vessels and lymphatic nodes. CCR7 expressed tumor cells can also metastasize to lymphatic system by the similar way as immune cells. Targeting CCL21/CCR7 axis to inhibit lymphatic metastasis but remain potent anti-tumor immune response has increasingly become a spot light of tumor immunotherapy. In this review, we summarize the role of CCL21/CCR7 axis in lymphatic metastasis, as well as preclinical trials and clinical trials in targeting CCL21/CCR7 axis for tumor metastasis therapy, hoping to accelerate the progress on tumor metastasis therapy by targeting CCL21/CCR7 axis.
Keywords: tumorlymphatic metastasisCCL21CCR7targeting therapy
恶性肿瘤严重威胁人类健康[1],而转移是大多数肿瘤患者死亡的主要原因[2-3]。淋巴转移往往是肿瘤转移的起始过程,肿瘤患者死亡主要是由癌细胞从原发位点扩散到淋巴结和远处器官所致[4]。趋化因子及其受体参与淋巴转移的起始过程,越来越多的研究表明CCL21/CCR7信号轴在淋巴转移中发挥关键作用[5-6]。CCL21主要由淋巴内皮细胞产生,其受体CCR7在DCs及T细胞表面均有表达。CCL21可通过与CCR7的相互作用控制DCs和T细胞等免疫细胞淋巴迁移及淋巴结归巢[7]。CCR7在乳腺癌[8]、食管鳞状细胞癌[9]、黑色素瘤[10]等多种恶性肿瘤细胞表面也有表达,且CCL21/CCR7信号轴参与调控肿瘤细胞迁移[11]。临床研究表明大多数肿瘤患者肿瘤组织中表达的CCR7与肿瘤转移密切相关[10, 12-15]。
近年来,靶向CCL21/CCR7轴治疗肿瘤转移的研究发展迅速。靶向CCL21/CCR7轴治疗肿瘤转移的临床前研究已在多种肿瘤模型中开展并取得较好的治疗效果[5, 16-18]。临床研究中多采用CCL21修饰过的DCs疫苗作为治疗策略。尽管基础与临床研究结果均提示CCL21/CCR7信号轴可作为肿瘤转移的潜在治疗靶标,靶向CCL21/CCR7轴治疗肿瘤转移的研究仍处于起步阶段。本文将基于靶向CCL21/CCR7轴治疗肿瘤转移的最新研究进展,对CCL21/CCR7信号轴在肿瘤转移中的作用及靶向CCL21/CCR7信号轴治疗肿瘤转移的临床前和临床研究进行重点介绍。
1 趋化因子及其受体趋化因子是一类由细胞分泌的分子量为7–12 kDa的细胞因子,其能够与相应受体结合从而发挥趋化细胞循其浓度作定向移动的生物学效应[19]。根据趋化因子多肽链近氨基端两个保守半胱氨酸残基的排列方式(图 1),可将其分为C、CC、CXC、CX3C四个亚家族[20]。趋化因子受体均属含7次跨膜结构的G蛋白偶联受体(G protein-coupled receptors, GPCRs),根据所结合的趋化因子类型的不同分为CR、CCR、CXCR、CX3CR四个亚家族[21]。趋化因子受体存在于所有的免疫细胞上,也经常存在于非免疫细胞上,如肿瘤细胞。趋化因子及其受体的主要作用是诱导细胞定向迁移,主要是免疫细胞的淋巴迁移及淋巴结归巢。此外,研究表明,趋化因子及其受体在肿瘤细胞侵袭和迁移中也扮演着重要的角色[22-23]。
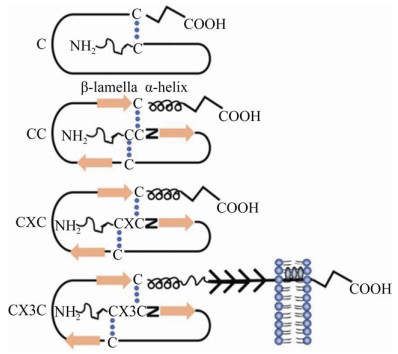 |
| 图 1 不同类型趋化因子的示意图(改自An等[24]) Fig. 1 Schematic diagram of different types of chemokines (adapted from An et al[24]). According to the arrangement of two conserved cysteine residues near the amino end of the peptide chain of chemokine, it can be divided into four subfamilies: C, CC, CXC and CX3C. |
| 图选项 |
2 CCL21及其受体CCR7的概述2.1 CCL21的概述CCL21主要由淋巴内皮细胞产生,其独特的长C末端可通过与糖胺聚糖(Glycosaminoglycan, GAGs)等结合将其固定到细胞外基质上或细胞表面[25]。结合到细胞表面的CCL21可形成淋巴管至周围组织由高到低的浓度梯度,并不断将细胞趋化至淋巴管[26]。与大多数趋化因子是在感染和炎症过程中诱导表达不同,CCL21是构成性表达的,并在特定空间形成浓度梯度[6]。CCL21在淋巴管分布最多,随着距离淋巴管距离的增加而逐渐降低[27]。有研究表明淋巴结内CCL21浓度梯度的形成与非典型趋化因子受体1 (Atypical chemokine receptor 1,CCRL1)有关。作为CCL21的非典型受体,CCRL1表达于淋巴结边缘,其主要在被膜下淋巴窦顶部(而不是底部)的淋巴内皮细胞表达[28-29],CCRL1能够结合并清除淋巴窦腔内的CCL21,从而形成淋巴结实质(淋巴结内部)到被膜下淋巴窦(淋巴结边缘)由高到低的CCL21浓度梯度[28, 30]。敲除CCRL1后,在不改变淋巴结CCL21表达总量的前提下可以逆转淋巴结局部CCL21的浓度梯度[28]。
2.2 CCR7的概述CCR7属GPCRs家族,是CCL21的唯一受体。人类CCR7共享G蛋白偶联受体的7个跨膜(TM1–TM7)螺旋折叠,螺旋由3个细胞内环和3个细胞外环连接,一个短的脂肪螺旋把CCR7锚定在细胞膜的细胞质侧[6]。CCR7在DCs、T细胞和B细胞等免疫细胞上均有表达,CCR7及其配体CCL21介导的信号控制DCs和T细胞等免疫细胞的淋巴迁移及淋巴结归巢[7, 31]。最近的研究证实CCR7在乳腺癌[8]、食管鳞状细胞癌[12]、黑色素瘤[32]、头颈癌[33]、口腔鳞状细胞癌[34]、非小细胞肺癌[35]、结直肠癌[36]、胃癌[37]等多种恶性肿瘤细胞表面呈过表达,这种过表达与肿瘤细胞的转移[38]及预后[39-41]密切相关。
2.3 CCL21与CCR7的相互作用CCL21与CCR7相互作用可在DCs中激活两个独立的信号轴(图 2),一个涉及调控趋化性的丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)家族成员,另一个涉及调节DCs迁移速度的Rho/Pyk2/Cofilin。CCL21/CCR7轴可通过激活MAPKs的下游信号ERK1/2,增强淋巴细胞的趋化性。当ERK1/2信号被抑制时,淋巴细胞的迁移能力降低。此外,ERK1/2可与JNK和p38通过非协同的方式调节CCR7介导的DCs趋化作用[42-44]。CCR7诱导GTP酶Rho、酪氨酸激酶Pyk2的活化和Cofilin的失活,干扰Rho或Pyk2可抑制Cofilin的失活和DCs的迁移速度。即使没有趋化因子的作用,干扰Rho/Pyk2/Cofilin也会抑制DCs迁移速度,这说明该信号轴可以控制DCs的迁移速度,CCR7通过活化其组分,导致迁移速度的提高[42]。扣留蛋白β (β-arrestin)作为GPCRs通路中的重要信号调节因子,能与G蛋白偶联受体激酶(G protein-coupled receptor kinases,GRKs)联合作用,调节受体内吞、信号转导及细胞凋亡等,参与细胞信号转导的级联反应[45-46]。在慢性淋巴细胞白血病中,衔接蛋白可以使CCR7以丝氨酸磷酸化蛋白的形式聚集内涵体中并与β-arrestin结合。当衔接蛋白缺失时,CCR7可以从与β-arrestin结合的内体中释放出来,进而促进CCR7的循环[47]。在胆固醇负荷的DCs中,抑制β-arrestin表达下调了CCR7的表达。比索洛尔(AR-β1阻滞剂)可抑制这一过程,从而促进DCs迁移[48]。此外,CCR7可以通过磷脂酰肌醇3-激酶(Phosphatidylinositol 3-kinase,PI3K)/蛋白激酶B (Protein kinase B,Akt)通路抑制成熟DCs的凋亡[49]。
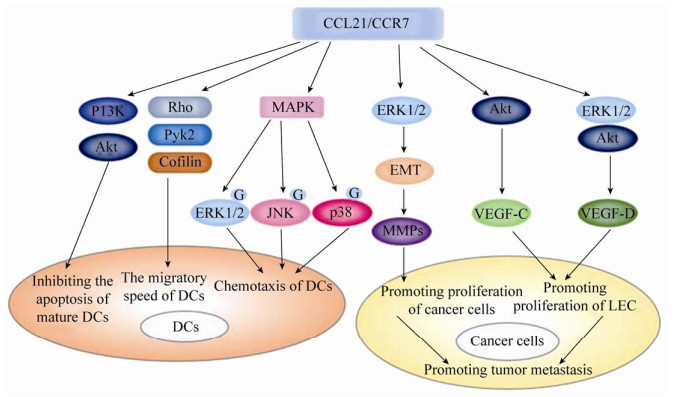 |
| 图 2 CCL21/CCR7轴在DCs和肿瘤细胞内的信号通路 Fig. 2 Cell signaling pathways of CCL21/CCR7 axis in DCs and tumor cells. CCL21/CCR7 axis inhibits the apoptosis of mature DCs by PI3K/Akt pathway. CCL21/CCR7 axis regulates chemotaxis of DCs by activating MAPK members ERK1/2, JNK, and p38. CCL21/CCR7 axis regulates the migratory speed of DCs by activating Rho and Pyk2, and inactivating cofilin. CCL21/CCR7 axis promotes proliferation of cancer cells by activating ERK1/2 pathway, inducing EMT of tumor cells and upregulating the expression of MMPs. CCL21/CCR7 axis could promotes proliferation of LEC by activating Akt pathway and Akt/ERK1/2 pathway of tumor cells. |
| 图选项 |
CCL21与CCR7结合后产生的细胞信号通路也直接参与肿瘤转移的过程(图 2)。CCL21/CCR7轴可激活ERK1/2信号通路,上调基质金属蛋白酶-2 (Matrix metalloproteinase-2,MMP-2)或基质金属蛋白酶-9 (Matrix metalloproteinase-9,MMP-9)的表达,促进膀胱癌细胞增殖,增强B淋巴细胞慢性白血病细胞(B-cell chronic lymphocytic leukemia cell,B-CLL)迁移和侵袭的能力[50-51]。MMPs可以降解和重塑细胞外基质(Extracellular matrix,ECM)或通过调控肿瘤微环境,促进肿瘤细胞的侵袭和迁移。MMPs通过细胞外周的蛋白水解作用重塑ECM,破坏ECM的物理屏障,引起肿瘤细胞侵袭和转移[52]。MMPs在肿瘤血管形成和淋巴管形成的过程中也发挥着重要作用。MMP-9能够提高瘤细胞对血管内皮生长因子(Vascular endothelial growth factor,VEGF)的生物利用率,促进新生血管的形成[53],也影响淋巴管形成,促进癌细胞向淋巴管内转移和散布[54]。CCL21可呈剂量依赖性地诱导ERK1/2磷酸化,诱导肺癌细胞上皮间质转化(Epithelial-mesenchymal transition,EMT),而抑制ERK1/2磷酸化可阻断CCL21/CCR7介导的肺癌细胞EMT[15]。此外,CCL21/CCR7轴可诱导ERK1/2磷酸化,磷酸化的ERK1/2能够上调血管内皮生长因子D (Vascular endothelial growth factor-D,VEGF-D)的表达,而VEGF-D表达上调可通过增强淋巴管的形成促进肿瘤转移[35, 55]。CCL21/CCR7信号轴也可以通过激活Akt信号通路上调肿瘤细胞中血管内皮生长因子C (Vascular endothelial growth factor-C,VEGF-C)的分泌和表达[8, 34]。综上,CCL21/CCR7信号轴可通过多种机制参与肿瘤转移。
3 CCL21/CCR7轴在肿瘤转移中的作用3.1 CCL21/CCR7轴对淋巴细胞的影响CCL21及其受体CCR7介导的信号控制T细胞和DCs等免疫细胞的淋巴迁移及淋巴结归巢[7, 56]。不同功能的T细胞亚群主要通过高内皮小静脉进入淋巴结,抗原负载的组织来源DCs主要通过传入淋巴管进入淋巴结[57],虽然两种细胞进入淋巴结的方式不同,但都与CCR7的表达有关[28]。T细胞从血液中向外周淋巴结迁移是一个多步骤的过程,首先淋巴细胞的L-选择素和PNAd (Peripheral?node addressins)结合,两者相互作用使T细胞快速且短暂附着在高内皮微静脉(High endothelial venules,HEVs)上,由于血液流动导致细胞滚动,滚动的淋巴细胞与通过GAGs锚定到细胞表面的CCL21结合,CCR7介导的信号与血液流动的剪切力诱导整合蛋白的构象变化,使它能够牢固地结合细胞间粘附分子1和2 (Intercellular cell adhesion molecule-1,intercellular cell adhesion molecule-2,ICAM1,ICAM2),淋巴细胞在内皮表面进行横向移动,然后进行跨内皮细胞迁移(图 3),这可能是由内皮细胞腔或腔外表面存在的CCL21诱导的[26]。研究表明CCL21的浓度梯度是CCR7介导的淋巴细胞淋巴结归巢的主要决定因素[28, 58-59],表达CCR7的细胞可沿着CCL21浓度由低到高的方向迁移[27]。组织来源的DCs先沿着CCL21的趋化性梯度进入初始的毛细淋巴管,这些毛细淋巴管通过集体性淋巴管将DCs向下引导到引流淋巴结的被膜下淋巴窦,经过细胞屏障到达淋巴结旁皮质[28]。骨髓来源的抑制细胞(Myeloid-derived suppressor cells,MDSCs)聚集于肿瘤及外周淋巴器官,通过抑制免疫反应促进肿瘤发展[60]。将CCL21基因敲除或过表达CCL21的黑色素瘤细胞移植到小鼠体内,肿瘤细胞产生的CCL21可招募更多的MDSCs和调节性T细胞至肿瘤组织,最终导致CCL21高表达的肿瘤生长体积明显大于CCL21低表达的肿瘤[61-62]。此外,有研究表明,CCR7配体可以增加成熟树突状细胞的抗原摄取能力[63]。
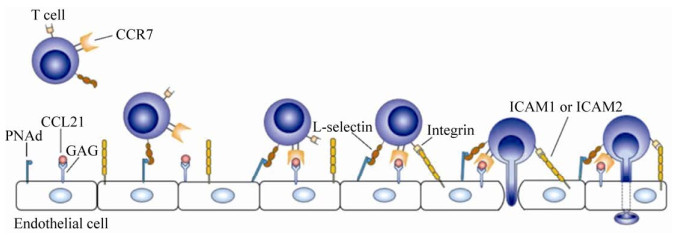 |
| 图 3 T细胞淋巴结归巢的示意图(改自F?rster等[26]) Fig. 3 The sketch map of T cells homing to lymph nodes (adapted from F?rster et al[26]). T cell migration from the blood to the peripheral lymph nodes is a multi-step process including tethering and rolling, CCR7-mediated activation, arrest and transmigration. |
| 图选项 |
3.2 CCL21/CCR7轴对肿瘤细胞的影响表达CCR7的肿瘤细胞同样可以感知来自淋巴管由高到低的CCL21梯度,沿着CCL21浓度由低到高的方向迁移进入淋巴管[64]。此外,肿瘤细胞在缓慢的组织间液流影响下,通过将CCR7配体分泌到细胞外基质ECM中,从而生成CCR7配体的自体梯度。这种机制利用淋巴管的排泄功能以趋化方式将肿瘤细胞导向服务于肿瘤的淋巴管,从而促进肿瘤细胞向功能性淋巴管迁移[64]。CCL21/CCR7信号轴具有控制肿瘤淋巴转移的作用[65],其也可以促进肿瘤细胞的生长和增殖[66-67]。研究表明,在CCL21作用下,A549-CCR7细胞的体外增殖、迁移和侵袭能力与肺腺癌A549-pEGFP细胞相比均显著增强[68]。此外,CCL21对T24细胞增殖也有促进作用且呈浓度依赖性,其中200 ng/mL浓度诱导的增殖量最大。CCL21/CCR7相互作用后MMP-2和MMP-9蛋白表达明显升高,促进膀胱癌T24细胞增殖并增强其迁移和侵袭能力。随着CCL21浓度的增加,Bcl-2蛋白表达呈上升趋势,Bax蛋白表达呈下降趋势,增加了T24细胞的抗凋亡作用[51, 69]。
4 靶向CCL21/CCR7轴治疗肿瘤转移的临床前研究干预CCL21/CCR7轴已成为肿瘤转移治疗的潜在靶点。临床前研究中,下调CCL21/CCR7轴或者增强CCL21/CCR7轴均可用于抗肿瘤治疗(表 1)。CCL21/CCR7轴可促进肿瘤细胞的淋巴转移,通过下调CCL21/CCR7轴可抑制肿瘤细胞转移[5]。另一方面,增加肿瘤微环境中CCL21,重塑肿瘤组织和肿瘤相关淋巴管之间CCL21的浓度梯度,可增强CCL21/CCR7轴,不仅可以进一步阻断CCR7介导的肿瘤细胞淋巴管迁移,还可使得肿瘤组织招募更多的免疫细胞发挥肿瘤细胞杀伤作用[16, 18]。CCL21/CCR7轴在肿瘤中的作用具有双向性:一方面表达于肿瘤细胞的CCR7与CCL21结合促进肿瘤的发展,另一方面表达于DCs和T细胞等免疫细胞的CCR7与CCL21结合抑制肿瘤的发展。CCL21/CCR7轴通过激活ERK1/2信号通路上调基质金属蛋白酶(Matrix metalloproteinases,MMPs)的表达,促进肿瘤细胞的增殖[51],同时也能上调血管内皮生长因子(Vascular endothelial growth factor,VEGF)的表达,诱导血管生成促进肿瘤转移[34-35]。表达CCR7的肿瘤细胞沿着CCL21浓度由低到高的方向迁移进入淋巴管进行淋巴转移[64]。而免疫细胞表达的CCR7与CCL21结合后通过调控T细胞和树突状细胞(Dendritic cells,DCs)等免疫细胞的淋巴迁移及淋巴结归巢,刺激免疫应答抑制肿瘤的发展[56]。
表 1 靶向CCL21-CCR7信号轴的临床前试验Table 1 Preclinical trials targeting CCL21-CCR7 axis
| Strategies | Interventions | Methods | Results | References |
| Down regulating CCL21/CCR7 interaction | CCL21 antibody | Combined with CCL21 | Decreasing CCL21/CCR7 interaction | [70] |
| CCR7 antibody | Combined with CCR7 | Decreasing CCL21/CCR7 interaction | [71] | |
| siRNA-CCR7 | Inhibiting expression of CCR7mRNA | Decreasing expression of CCR7 | [50, 72–73] | |
| CCR7 trap | Targeted delivery to tumor | Decreasing CCL21/CCR7 interaction | [5] | |
| CCR7 antagonist | Combined with CCR7 | Inactivation of CCR7 | [6, 74] | |
| CCL21 mutant | Combined with CCR7 | Decreasing CCL21/CCR7 interaction | [27, 75] | |
| Enhancing CCL21/CCR7 interaction | Intratumoral injection of CCL21 | Reshaping CCL21gradient | Blocking CCR7-mediated tumor metastasis; Recruiting immune cells | [16] |
| Construction of cancer cells of expressing CCL21 | Reshaping CCL21gradient | Blocking CCR7-mediated tumor metastasis; Recruiting immune cells | [18] |
表选项
4.1 下调CCL21/CCR7轴的手段及研究结果下调CCL21/CCR7轴的策略包括靶向CCL21的CCL21中和抗体和靶向CCR7的CCR7中和抗体、siRNA-CCR7、CCR7 trap、CCR7拮抗剂和CCL21突变体等(图 4)。
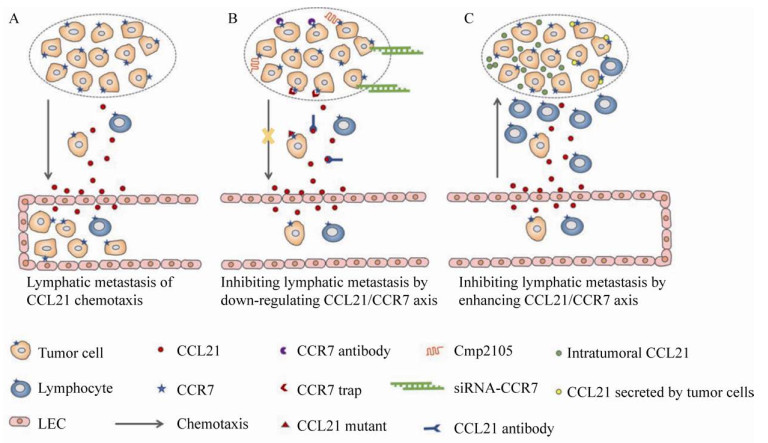 |
| 图 4 靶向CCL21/CCR7信号轴治疗肿瘤转移的策略 Fig. 4 Treatment strategies of tumor metastasis targeting CCL21/CCR7 axis. (A) Mechanism of CCL21/CCR7 axis controlling tumor cells lymph node metastasis and lymphocytes lymph node homing. (B) Treatment strategies of down-regulating CCL21/CCR7 axis to block the metastasis of tumor cells into lymphatic system. (C) Enhancing CCL21/CCR7 axis could block the metastasis of tumor cells into lymphatic system by recruiting more immune cells. |
| 图选项 |
CCL21/CCR7相互作用可以诱导肿瘤细胞的迁移和侵袭,而抗CCL21抗体可以通过和CCR7竞争性结合CCL21从而阻断这种作用。Wiley等[70]发现CCL21的中和抗体通过阻断CCL21作用阻止肿瘤细胞的淋巴转移。有研究指出,抗CCR7抗体治疗可以有效减少肿瘤细胞的迁移和侵袭。通过皮下或静脉接种人淋巴瘤细胞至小鼠体内,测试抗CCR7抗体疗法。与未治疗的小鼠相比,CCR7抗体治疗明显延迟了皮下肿瘤的出现,抑制了肿瘤的生长速度。此外,CCR7抗体处理的小鼠与未处理的小鼠相比,肿瘤转移到远处淋巴器官的肿瘤数量显著减少且凋亡肿瘤细胞数量显著增加。在静脉注射模型中,CCR7抗体治疗显著提高了小鼠的存活率[71]。上述研究结果表明CCR7抗体具有显著的抗肿瘤转移效果。siRNA-CCR7可以在mRNA水平上抑制CCR7的表达[72]。在B-CLL中,CCL21/CCR7轴激活ERK1/2信号通路,上调MMP-9的表达,从而促进B-CLL细胞的迁移。CCR7-siRNA可通过抑制CCR7的表达来减少CCL21/CCR7结合从而阻断B-CLL的迁移[50]。Yu等[73]通过体外实验发现,siRNA-CCR7可以抑制结肠癌SW620细胞的侵袭,并抑制其淋巴结转移。将表达CCR7拮抗蛋白的基因递送系统靶向递送至肿瘤部位后,肿瘤细胞表达的CCR7 trap分泌到细胞外与肿瘤细胞表面CCR7结合能够阻断CCL21/CCR7介导的肿瘤细胞淋巴迁移[5]。
CCR7本质上是变构性蛋白,通过调控7次跨膜结构域的胞内、胞外段实现信号传导功能。开发小分子药物靶向CCR7或许是抑制肿瘤淋巴转移的理想策略。小分子Cmp2105可增强CCR7的热稳定性,同时可结合CCR7使CCR7稳定在失活的构象。结构学分析显示Cmp2105与CCR7的变构结构口袋的胞内段结合,抑制CCR7的活化[6, 74]。研究发现在变异结合口袋中保守的TM7-H8结构域是竞争性配体结合的关键位点,因此TM7-H8结构域可能是抑制趋化因子受体的靶向位点,然而小分子化合物靶向CCL21/CCR7轴的生物学效应有待于进一步研究。C端截短突变体CCL21可以和CCL21竞争性地结合CCR7却不激活CCR7下游的信号传导系统,从而阻断CCL21引起的细胞迁移,同时其能够阻断CCL21介导的ERK磷酸化,抑制CCL21对人结肠癌细胞SW480的趋化作用[75]。C端截短突变体CCL21会误导DCs的迁移,最终使DCs分散在远离淋巴管的位置[27]。
4.2 增强CCL21/CCR7轴的手段及研究结果如前所述,CCL21/CCR7轴对淋巴细胞和肿瘤细胞产生作用,增强CCL21/CCR7轴对淋巴细胞的影响已用于肿瘤转移的治疗研究(图 4)。瘤内注射CCL21会导致肿瘤组织招募的T淋巴细胞和DCs显著增加。同时瘤内注射CCL21可以使淋巴细胞的溶细胞能力增强,这些发现为进一步评价CCL21在肿瘤免疫中的作用及其在抗肿瘤治疗中的应用提供了有力的证据[16]。有研究者构建了表达CCL21的小鼠前列腺癌细胞并将其接种至小鼠体内,发现肿瘤微环境中的过量的CCL21可以同时抑制肿瘤的原位生长和远处转移,延长荷瘤小鼠的生存期[18]。
笔者团队致力于研究炎症性细胞因子,如干扰素γ (Interferon gamma,IFNγ)、肿瘤坏死因子(Tumor necrosis factor,TNF)、白细胞介素4 (Interleukin 4,IL4)等细胞因子在肿瘤发生、发展及排斥过程中的作用,尤其是对肿瘤微环境形成和肿瘤间质细胞功能的影响。2000年,笔者团队就提出IFNγ依赖的抗血管新生是肿瘤免疫排斥的主要机制[76],近年来在细胞因子参与肿瘤发生、发展及肿瘤靶向治疗领域发表一系列文章[77-84]。基于研究团队已有的研究工作,简要分析目前靶向CCL21/CCR7轴手段的优势与不足之处及可能的研究方向。
使用CCR7的抗体时,由于缺乏组织特异性,CCR7抗体可引起免疫抑制副作用,而通过靶向递送技术,将表达CCR7 trap的纳米制剂靶向递送至肿瘤组织,可通过下调CCL21/CCR7轴抑制肿瘤细胞转移且不会产生免疫抑制副作用[5]。因此通过纳米或者其他技术手段增强CCR7抗体的组织特异性或将有助于进一步推动靶向CCL21/CCR7轴治疗肿瘤相关研究的进展[5, 71]。siRNA-CCR7在mRNA水平上抑制了CCR7的表达,更早地从根本上阻止了CCL21/CCR7介导的肿瘤转移。CCR7 trap则利用纳米技术将表达CCR7拮抗蛋白的基因递送到肿瘤组织,使其更加准确地到达肿瘤部位,增加了临床应用的可能性。小分子Cmp2105可以结合CCR7使其失活,但小分子化合物靶向CCL21/CCR7轴的生物学效应目前还不清楚。C端截断突变体CCL21可以竞争性抑制CCL21,同时能够阻断CCL21介导的ERK磷酸化,但其作用机制目前还不清楚。C端截短突变体CCL21会误导DCs的迁移,最终影响DCs的淋巴结归巢。
瘤内注射CCL21和接种表达CCL21的肿瘤细胞这两种方法对抗肿瘤治疗有一定的影响,即便如此,研究过程中仍存在一些需要解决的问题和可以改进的方面。比如,瘤内注射的CCL21是否可以稳定地存在于肿瘤组织内,是否可以模拟体内CCL21锚定到细胞表面进行仿生设计将CCL21锚定到肿瘤组织上?对于接种表达CCL21的肿瘤细胞这种方法,是否可以利用纳米技术将CCL21或者表达CCL21的DNA/mRNA递送到肿瘤组织增加其临床应用的可能性?
5 靶向CCL21/CCR7轴治疗肿瘤转移的临床试验研究至2020年3月,clinicaltrials.gov的最新数据显示,靶向CCL21/CCR7治疗肿瘤的临床试验仅有5项[85]。在已开展的5项临床研究中,4项是关于肺癌的研究,1项是关于黑色素瘤的研究(表 2)。在CCL21基因修饰的DCs联合帕博利珠单抗治疗晚期非小细胞肺癌的1期临床试验中,主要通过使用不同浓度CCL21基因修饰的DCs和帕博利珠单抗联合确定其使用时的安全性和最大耐受量。受试者第0、21、42天瘤内注射CCL21基因修饰的DCs,1 h后再静脉注射帕博利珠单抗,3次瘤内注射后,每3周接受一次帕博利珠单抗治疗至一年。对于肺癌和腺癌,GM.CD40L.CCL21疫苗的1期和2期临床试验正在逐步展开。肺癌细胞和经基因改造后表达GM-CSF和CD40L的细胞混合后经过高剂量X射线照射,旨在通过增强机体的免疫反应更好地发挥体内抗肿瘤效能。1期临床试验主要通过皮内注射不同剂量的疫苗来确定最大耐受量,2期临床试验主要通过和GM.CD40L细胞疫苗进行比较,确定GM.CD40L.CCL21疫苗的有效性。对于Ⅲ B期、Ⅳ期和复发的非小细胞肺癌,受试者注射表达CCL21的DCs进行1期临床试验来研究该疫苗在治疗Ⅲ B期、Ⅳ期和复发的非小细胞癌的副作用和最适剂量以及最大耐受量和毒性。对于黑色素瘤,CCL21基因改造的树突状细胞疫苗也正在进行1期临床试验。总体来讲,靶向CCL21/CCR7轴治疗肿瘤转移的临床试验尚处于起步阶段。
表 2 靶向CCL21-CCR7信号轴的临床试验Table 2 Clinical trials of targeting CCL21-CCR7 axis
| Conditions | Interventions | Drug-delivery way | NCT number |
| Carcinoma, Non-Small-Cell lung | Genetic:Ad-CCL21-DC Drug: Pembrolizumab | Intratumoral injection Intravenous injection | NCT03546361 |
| Lung cancer adenocarcinoma | GM.CD40L.CCL21 vaccinations GM.CD40L cells vaccinations | Intradermal injection | NCT01433172 |
| Melanoma (Skin) | Autologous dendritic cell-adenovirus CCL21 vaccine | Intradermal injection | NCT00798629 |
| Lung cancer | Autologous dendritic cell-adenovirus CCL21 vaccine | Intratumoral injection | NCT00601094 |
| NSCLC | Autologous dendritic cell-adenovirus CCL21 vaccine | Intratumoral injection | NCT01574222 |
| Source: https://clinicaltrials.gov/. | |||
表选项
6 总结与展望在经历近30年的基础研究后,CCL21/CCR7轴在肿瘤领域的研究发展迅速[6, 8, 34, 86]。早期的CCL21/CCR7轴研究大多集中于其在淋巴细胞迁移及肿瘤发生和转移中的作用,CCL21/CCR7轴目前已在临床前研究中用于三阴性乳腺癌转移的治疗[5, 42, 64],此外黑色素瘤和肺癌等恶性肿瘤的临床防治研究也在逐步开展。尽管发展势头良好,靶向CCL21/CCR7轴治疗肿瘤的临床研究多使用CCL21基因工程修饰过的DCs,且处于起步阶段(尚未查到国内在这方面的临床试验)[85]。事实上,基因工程改造的DCs疫苗在肿瘤领域的应用已经取得了长足的进展。FDA批准的DCs肿瘤疫苗[87]和最近靶向CCL21/CCR7的临床试验,为DCs作为载体靶向CCL21/CCR7信号轴的有效性和安全性提供了越来越多的证据。
直接靶向CCL21/CCR7轴治疗肿瘤转移已在临床前研究中表现出一定的应用潜力[5, 27, 73],实际上与CCL21/CCR7轴密切联系的上游与下游事件比如EMT、MMP-2/MMP-9表达、T细胞淋巴结归巢,CCL21浓度梯度调节等均有可能用于肿瘤转移的靶向治疗[88-92]。肿瘤转移是一个复杂的生物学过程,联合上述2种或者多种治疗手段理论上可通过不同的机制抑制肿瘤转移,或许可以达到意想不到的效果。此外,有研究表明化疗作为肿瘤的主要治疗策略,具有促进肿瘤转移的作用[93-95]。若在对肿瘤进行化疗时,联合抑制肿瘤转移的治疗策略,如通过CCR7 trap下调肿瘤细胞的淋巴迁移能力或者通过调节CCL21浓度梯度增强肿瘤组织招募淋巴细胞的作用有可能进一步改善肿瘤的治疗现状。
在临床前研究工作中,限制靶向CCL21/CCR7轴治疗肿瘤转移的主要问题在于如何靶向CCL21/CCR7轴,既能抑制淋巴转移,又不影响抗肿瘤免疫反应。利用靶向递送技术,将CCR7中和抗体、CCR7小分子拮抗剂、CC21中和抗体及CCL21突变体等下调肿瘤细胞迁移能力的候选药物或者CCL21等上调肿瘤组织招募淋巴细胞的候选药物特异性递送至肿瘤组织,将对CCL21/CCR7轴的干预局限在肿瘤组织局部或可解决上述问题。在深入了解CCL21/CCR7轴在肿瘤转移过程中起到作用的基础上,设计靶向治疗药物正逐步成为CCL21/CCR7轴研究的发展方向与趋势,并将进一步促进肿瘤转移治疗药物的研发进展。
在临床研究工作中,受试者的纳入标准、用药剂量、用药周期及是否联合其他治疗手段,均可能影响临床研究的结果。目前临床前研究的CCR7中和抗体、siRNA-CCR7、利用纳米技术靶向递送的CCR7 trap、CCR7的小分子拮抗剂和CCL21中和抗体等经过进一步研究均有可能进入临床研究阶段。合理的临床试验设计可以提高靶向CCL21/CCR7信号轴治疗肿瘤转移的有效性和安全性,而靶向CCL21/CCR7信号轴抗肿瘤积极的临床试验结果在更多的患者中再得到进一步验证,将进一步增强研究人员对靶向CCL21/CCR7信号轴抗肿瘤有效性和安全性的信心。深入研究CCL21/CCR7轴在肿瘤转移中的作用机制,开发靶向CCL21/CCR7轴的治疗策略和更多、更合理的临床试验等均将促进靶向CCL21/CCR7轴在肿瘤转移防治领域迅速发展。
参考文献
| [1] | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin, 2020, 70(1): 7-30. DOI:10.3322/caac.21590 |
| [2] | Guan XM. Cancer metastases: challenges and opportunities. Acta Pharm Sin B, 2015, 5(5): 402-418. DOI:10.1016/j.apsb.2015.07.005 |
| [3] | Jyotsana N, Zhang ZJ, Himmel LE, et al. Minimal dosing of leukocyte targeting TRAIL decreases triple-negative breast cancer metastasis following tumor resection. Sci Adv, 2019, 5(7): eaaw4197. DOI:10.1126/sciadv.aaw4197 |
| [4] | Zheng ZX, Zhang YN, Zhang LH, et al. A nomogram for predicting the likelihood of lymph node metastasis in early gastric patients. BMC Cancer, 2016, 16: 92. DOI:10.1186/s12885-016-2132-5 |
| [5] | An S, Tiruthani K, Wang Y, et al. Locally trapping the C-C chemokine receptor type 7 by gene delivery nanoparticle inhibits lymphatic metastasis prior to tumor resection. Small, 2019, 15(9): 1805182. DOI:10.1002/smll.201805182 |
| [6] | Jaeger K, Bruenle S, Weinert T, et al. Structural basis for allosteric ligand recognition in the human CC chemokine receptor 7. Cell, 2019, 178(5): 1222-1230. DOI:10.1016/j.cell.2019.07.028 |
| [7] | Braun A, Worbs T, Moschovakis GL, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol, 2011, 12(9): 879-887. DOI:10.1038/ni.2085 |
| [8] | Tutunea-Fatan E, Majumder M, Xin XP, et al. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer, 2015, 14: 35. DOI:10.1186/s12943-015-0306-4 |
| [9] | Shi M, Chen D, Yang D, et al. CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer Res, 2015, 34: 149. DOI:10.1186/s13046-015-0268-9 |
| [10] | Emmett MS, Lanati S, Dunn DBA, et al. CCR7 mediates directed growth of melanomas towards lymphatics. Microcirculation, 2011, 18(3): 172-182. DOI:10.1111/j.1549-8719.2010.00074.x |
| [11] | Tejchman A, Lamerant-Fayel N, Jacquinet JC, et al. Tumor hypoxia modulates podoplanin/CCL21 interactions in CCR7+ NK cell recruitment and CCR7+ tumor cell mobilization. Oncotarget, 2017, 8(19): 31876-31887. DOI:10.18632/oncotarget.16311 |
| [12] | Irino T, Takeuchi H, Matsuda S, et al. CC-Chemokine receptor CCR7: a key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer, 2014, 14: 291. DOI:10.1186/1471-2407-14-291 |
| [13] | Li X, Sun S, Li N, et al. High expression of CCR7 predicts lymph node metastasis and good prognosis in triple negative breast cancer. Cell Physiol Biochem, 2017, 43(2): 531-539. |
| [14] | Xiong Y, Huang F, Li XZ, et al. CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer. Int J Oncol, 2017, 51(1): 75-90. |
| [15] | Zhong GX, Chen L, Yin RH, et al. Chemokine (C-C motif) ligand 21/C-C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Mol Med Rep, 2017, 15(6): 4100-4108. DOI:10.3892/mmr.2017.6534 |
| [16] | Sharma S, Stolina M, Luo J, et al. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol, 2000, 164(9): 4558-4563. DOI:10.4049/jimmunol.164.9.4558 |
| [17] | Zhou S, Chen LH, Zhen ZW, et al. Depletion of CD4+ CD25+ regulatory T cells promotes CCL21-mediated antitumor immunity. PLoS ONE, 2013, 8(9): e73952. DOI:10.1371/journal.pone.0073952 |
| [18] | Yousefieh N, Hahto SM, Stephens AL, et al. Regulated expression of CCL21 in the prostate tumor microenvironment inhibits tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Microenviron, 2009, 2(1): 59-67. DOI:10.1007/s12307-009-0021-z |
| [19] | Coniglio SJ. Role of Tumor-derived chemokines in osteolytic bone metastasis. Front Endocrinol (Lausanne), 2018, 9: 313. DOI:10.3389/fendo.2018.00313 |
| [20] | Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol, 2015, 93(4): 372-383. DOI:10.1038/icb.2015.15 |
| [21] | Gustavsson M. New insights into the structure and function of chemokine receptor: chemokine complexes from an experimental perspective. J Leukoc Biol, 2020. DOI:10.1002/JLB.2MR1219-288R |
| [22] | Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res, 2014, 2(12): 1125-1131. DOI:10.1158/2326-6066.CIR-14-0160 |
| [23] | Li BH, Garstka MA, Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol, 2020, 117: 201-215. DOI:10.1016/j.molimm.2019.11.014 |
| [24] | An YQ, Yao Z, Li DJ, et al. Medical Immunology. Beijing: Peking University Medical Press, 2013, 49-58 (in Chinese). 安云庆, 姚智, 李殿俊, 等. 医学免疫学. 北京:北京大学医学出版社, 2013, 49-58. |
| [25] | Yoshida R, Nagira M, Kitaura M, et al. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem, 1998, 273(12): 7118-7122. DOI:10.1074/jbc.273.12.7118 |
| [26] | F?rster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol, 2008, 8(5): 362-371. DOI:10.1038/nri2297 |
| [27] | Weber M, Hauschild R, Schwarz J, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science, 2013, 339(6117): 328-332. DOI:10.1126/science.1228456 |
| [28] | Ulvmar MH, Werth K, Braun A, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol, 2014, 15(7): 623-630. DOI:10.1038/ni.2889 |
| [29] | Heinzel K, Benz C, Bleul CC. A silent chemokine receptor regulates steady-state leukocyte homing in vivo. Proc Natl Acad Sci USA, 2007, 104(20): 8421-8426. DOI:10.1073/pnas.0608274104 |
| [30] | Bachelerie F, Graham GJ, Locati M, et al. New nomenclature for atypical chemokine receptors. Nat Immunol, 2014, 15(3): 207-208. DOI:10.1038/ni.2812 |
| [31] | Sadeghian-Rizi T, Khanahmad H, Jahanian-Najafabadi A. Therapeutic targeting of chemokines and chemokine receptors in multiple sclerosis: opportunities and challenges. CNS Neurol Disord Drug Targets, 2018, 17(7): 496-508. DOI:10.2174/1871527317666180713111100 |
| [32] | Cristiani CM, Turdo A, Ventura V, et al. Accumulation of circulating CCR7+ natural killer cells marks melanoma evolution and reveals a CCL19-dependent metastatic pathway. Cancer Immunol Res, 2019, 7(5): 841-852. DOI:10.1158/2326-6066.CIR-18-0651 |
| [33] | Wang J, Seethala RR, Zhang Q, et al. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst, 2008, 100(7): 502-512. DOI:10.1093/jnci/djn059 |
| [34] | Chen Y, Shao Z, Jiang EH, et al. CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J Cell Physiol, 2020, 235(9): 5995-6009. DOI:10.1002/jcp.29525 |
| [35] | Sun LM, Zhang QF, Li Y, et al. CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells. Int J Clin Exp Pathol, 2015, 8(12): 15729-15738. |
| [36] | Jiao XL, Shu GM, Liu H, et al. The diagnostic value of chemokine/chemokine receptor pairs in hepatocellular carcinoma and colorectal liver metastasis. J Histochem Cytochem, 2019, 67(5): 299-308. DOI:10.1369/0022155418824274 |
| [37] | Mashino K, Sadanaga N, Yamaguchi H, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res, 2002, 62(10): 2937-2941. |
| [38] | Jin S, Liu MD, Wu H, et al. Overexpression of hsa-miR-125a-5p enhances proliferation, migration and invasion of head and neck squamous cell carcinoma cell lines by upregulating C-C chemokine receptor type 7. Oncol Lett, 2018, 15(6): 9703-9710. |
| [39] | Du PZ, Liu YC, Ren H, et al. Expression of chemokine receptor CCR7 is a negative prognostic factor for patients with gastric cancer: a meta-analysis. Gastric Cancer, 2017, 20(2): 235-245. DOI:10.1007/s10120-016-0602-8 |
| [40] | González-Arriagada WA, Lozano-Burgos C, Zú?iga-Moreta R, et al. Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J Oral Pathol Med, 2018, 47(8): 755-763. DOI:10.1111/jop.12736 |
| [41] | Ma L, Kuai WX, Qi HX, et al. Expression of CCR7 and Tim-3 in childhood patients with acute lymphoblastic leukemia and their predictive value for prognosis. J Exp Hematol, 2019, 27(6): 1728-1735 (in Chinese). 马丽, 蒯文霞, 祁海啸, 等. CCR7与Tim-3在儿童急性淋巴细胞白血病中的表达及其联合检测对预后评估的价值. 中国实验血液学杂志, 2019, 27(6): 1728-1735. |
| [42] | Riol-Blanco L, Sánchez-Sánchez N, Torres A, et al. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol, 2005, 174(7): 4070-4080. DOI:10.4049/jimmunol.174.7.4070 |
| [43] | Puig-Kr?ger A, Relloso M, Fernández-Capetillo O, et al. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood, 2001, 98(7): 2175-2182. DOI:10.1182/blood.V98.7.2175 |
| [44] | Li GL, Basu S, Han MK, et al. Influence of ERK activation on decreased chemotaxis of mature human cord blood monocyte-derived dendritic cells to CCL19 and CXCL12. Blood, 2007, 109(8): 3173-3176. DOI:10.1182/blood-2006-04-014753 |
| [45] | Rosanò L, Cianfrocca R, Masi S, et al. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA, 2009, 106(8): 2806-2811. DOI:10.1073/pnas.0807158106 |
| [46] | Bagnato A, Rosano L. New routes in GPCR/beta-arrestin-driven signaling in cancer progression and metastasis. Front Pharmacol, 2019, 10: 114. DOI:10.3389/fphar.2019.00114 |
| [47] | Patrussi L, Capitani N, Cattaneo F, et al. p66Shc deficiency enhances CXCR4 and CCR7 recycling in CLL B cells by facilitating their dephosphorylation- dependent release from beta-arrestin at early endosomes. Oncogene, 2018, 37(11): 1534-1550. DOI:10.1038/s41388-017-0066-2 |
| [48] | Yang H, Du RZ, Qiu JP, et al. Bisoprolol reverses epinephrine-mediated inhibition of cell emigration through increases in the expression of beta-arrestin 2 and CCR7 and PI3K phosphorylation, in dendritic cells loaded with cholesterol. Thromb Res, 2013, 131(3): 230-237. DOI:10.1016/j.thromres.2012.12.009 |
| [49] | Sánchez-Sánchez N, Riol-Blanco L, De La Rosa G, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood, 2004, 104(3): 619-625. |
| [50] | Redondo-Mu?oz J, Terol MJ, García-Marco JA, et al. Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration. Blood, 2008, 111(1): 383-386. |
| [51] | Mo M, Zhou M, Wang L, et al. CCL21/CCR7 enhances the proliferation, migration, and invasion of human bladder cancer T24 cells. PLoS ONE, 2015, 10(3): e0119506. DOI:10.1371/journal.pone.0119506 |
| [52] | Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem, 2016, 31(sup1): 177-183. |
| [53] | Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell, 2008, 13(3): 193-205. |
| [54] | Bruyère F, Melen-Lamalle L, Blacher S, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods, 2008, 5(5): 431-437. |
| [55] | Li X, Wang XM, Li ZT, et al. Chemokine receptor 7 targets the vascular endothelial growth factor via the AKT/ERK pathway to regulate angiogenesis in colon cancer. Cancer Med, 2019, 8(11): 5327-5340. |
| [56] | Worbs T, Mempel TR, B?lter J, et al. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med, 2007, 204(3): 489-495. |
| [57] | Worbs T, F?rster R. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol, 2007, 28(6): 274-280. |
| [58] | Jafarnejad M, Zawieja DC, Brook BS, et al. A novel computational model predicts key regulators of chemokine gradient formation in lymph nodes and site-specific roles for CCL19 and ACKR4. J Immunol, 2017, 199(7): 2291-2304. |
| [59] | Randolph GJ, Ivanov S, Zinselmeyer BH, et al. The lymphatic system: integral roles in immunity. Annu Rev Immunol, 2017, 35: 31-52. |
| [60] | Yanagawa Y, Onoé K. CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities. Blood, 2003, 101(12): 4923-4929. |
| [61] | Kodumudi KN, Woan K, Gilvary DL, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res, 2010, 16(18): 4583-4594. |
| [62] | Li BH, Garstka MA, Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol, 2020, 117: 201-215. |
| [63] | Shields JD, Kourtis IC, Tomei AA, et al. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science, 2010, 328(5979): 749-752. |
| [64] | Shields JD, Fleury ME, Yong C, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell, 2007, 11(6): 526-538. |
| [65] | Maolake A, Izumi K, Natsagdorj A, et al. Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR 7 upregulation. Cancer Sci, 2018, 109(5): 1524-1531. |
| [66] | Wu J, Li L, Liu JN, et al. CC chemokine receptor 7 promotes triple-negative breast cancer growth and metastasis. Acta Biochim Biophys Sin (Shanghai), 2018, 50(9): 835-842. |
| [67] | Peng W, Dong N, Wu SH, et al. miR-4500 suppresses cell proliferation and migration in bladder cancer via inhibition of STAT3/CCR7 pathway. J Cell Biochem, 2019. DOI:10.1002/jcb.29558 |
| [68] | Guo XG. CCL21/CCR7 axis promotes A549 cell proliferation, migration, invasion in vitro and lymph node metastasis in vivo[D]. Chongqing: Third Military Medical University, 2007 (in Chinese). 郭学光. CCL21/CCR7轴对肺腺癌A549细胞增殖、迁移、侵袭及淋巴结转移的影响研究[D].重庆: 第三军医大学, 2007. |
| [69] | Zhou M, Yu WW, Wang S, et al. The influence of CCL21/CCR7 axis on T24 cells' biologic behavior. J Urol Clinic: Electr Vers, 2016, 8(1): 17-23 (in Chinese). 周密, 俞蔚文, 王帅, 等. CCL21/CCR7对T24细胞生物学行为的影响. 泌尿外科杂志:电子版, 2016, 8(1): 17-23. |
| [70] | Wiley HE, Gonzalez EB, Maki W, et al. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst, 2001, 93(21): 1638-1643. |
| [71] | Somovilla-Crespo B, Alfonso-Pérez M, Cuesta-Mateos C, et al. Anti-CCR7 therapy exerts a potent anti-tumor activity in a xenograft model of human mantle cell lymphoma. J Hematol Oncol, 2013, 6: 89. |
| [72] | Xu B, Zhou MJ, Qiu WC, et al. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial-mesenchymal transition and suppressing apoptosis through AKT pathway. Cancer Med, 2017, 6(5): 1062-1071. |
| [73] | Yu SY, Duan JP, Zhou ZQ, et al. A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biol Ther, 2008, 7(7): 1037-1043. |
| [74] | Saha S, Shukla AK. The inside story: crystal structure of the chemokine receptor CCR7 with an intracellular allosteric antagonist. Biochemistry, 2020, 59(1): 12-14. |
| [75] | Zhang S, Zhang G, Wang HS, et al. mCCL21-a(1-57), a C-terminal deletion mutant of CCL21, inhibits CCL21-mediated migration in cultured SW480 cells by suppressing ERK activation. Chin J Biochem Mole Biol, 2011, 27(2): 161-167 (in Chinese). 张淑, 张革, 王红胜, 等. C端截短突变体mCCL21-a(1-57)可拮抗CCL21介导的ERK磷酸化及细胞迁移. 中国生物化学与分子生物学报, 2011, 27(2): 161-167. |
| [76] | Qin ZH, Blankenstein T. CD4+ T Cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFNγ receptor expression by nonhematopoietic cells. Immunity, 2000, 12(6): 677-686. |
| [77] | Ni C, Ma P, Qu LW, et al. Accelerated tumour metastasis due to interferon-γ receptor-mediated dissociation of perivascular cells from blood vessels. J Pathol, 2017, 242(3): 334-346. |
| [78] | Li YA, Wang J, Song K, et al. S100A4 promotes hepatocellular carcinogenesis by intensifying fibrosis-associated cancer cell stemness. Oncoimmunology, 2020, 9(1): 1725355. |
| [79] | Zhang JH, Song K, Wang J, et al. S100A4 blockage alleviates agonistic anti-CD137 antibody-induced liver pathology without disruption of antitumor immunity. Oncoimmunology, 2018, 7(4): e1296996. |
| [80] | Ni C, Ma P, Wang RR, et al. Doxorubicin-induced cardiotoxicity involves IFNγ-mediated metabolic reprogramming in cardiomyocytes. J Pathol, 2019, 247(3): 320-332. |
| [81] | Atretkhany KSN, Nosenko MA, Gogoleva VS, et al. TNF neutralization results in the delay of transplantable tumor growth and reduced MDSC accumulation. Front Immunol, 2016, 7: 147. |
| [82] | Ji TJ, Lang JY, Ning B, et al. Enhanced natural killer cell immunotherapy by rationally assembling Fc fragments of antibodies onto tumor membranes. Adv Mater, 2019, 31(6): 1804395. |
| [83] | Zhang LJ, Qi YQ, Min H, et al. Cooperatively responsive peptide nanotherapeutic that regulates angiopoietin receptor Tie2 activity in tumor microenvironment to prevent breast tumor relapse after chemotherapy. ACS Nano, 2019, 13(5): 5091-5102. |
| [84] | Ji TJ, Lang JY, Wang J, et al. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy. ACS Nano, 2017, 11(9): 8668-8678. |
| [85] | U.S. National Library of Medicine: ClinicalTrials. gov.[2020-07-05]. https://clinicaltrials.gov/ct2/results?cond=&term=CCL21&cntry=&state=&city=&dist=. |
| [86] | Ju YH, Sun CZ, Wang XL. Loss of atypical chemokine receptor 4 facilitates C-C motif chemokine ligand 21-mediated tumor growth and invasion in nasopharyngeal carcinoma. Exp Ther Med, 2019, 17(1): 613-620. |
| [87] | Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol, 2009, 27(2): 129-139. |
| [88] | Mohanty R, Chowdhury CR, Arega S, et al. CAR T cell therapy: A new era for cancer treatment (Review). Oncol Rep, 2019, 42(6): 2183-2195. |
| [89] | Du BW, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules, 2016, 21(7): 965. |
| [90] | Singh M, Yelle N, Venugopal C, et al. EMT: Mechanisms and therapeutic implications. Pharmacol Ther, 2018, 182: 80-94. |
| [91] | Wang XF, Nagase H, Watanabe T, et al. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci, 2010, 101(3): 759-766. |
| [92] | Liu T, Wu XD, Wang YG, et al. CD-PLLD co-delivering docetaxel and MMP-9 siRNA plasmid for nasopharyngeal carcinoma therapy in vivo. Mol Med Rep, 2017, 16(2): 1383-1388. |
| [93] | Karagiannis GS, Pastoriza JM, Wang YR, et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med, 2017, 9(397): eaan0026. |
| [94] | Keklikoglou I, Cianciaruso C, Gü? E, et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol, 2019, 21(2): 190-202. |
| [95] | D'Alterio C, Scala S, Sozzi G, et al. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin Cancer Biol, 2020, 60: 351-361. |
