
 , 李晶1,2
, 李晶1,2

1. 中国科学院微生物研究所 中国科学院病原微生物与免疫学重点实验室,北京 100101;
2. 中国科学院大学,北京 100049;
3. 北京海淀外国语实验学校,北京 100195
收稿日期:2018-11-28;接收日期:2019-03-19
基金项目:国家重点研发计划(No.2017YFD051105), 中国科学院青年促进人才项目(No.2019091)资助
摘要:为研究口服鸡α干扰素最佳的给药频率及与灭活病毒联合使用对鸡群的影响,文中将构建的原核表达质粒pET-22b-ChIFN-α转入大肠杆菌Escherichia coli BL21 (DE3),宿主菌经诱导表达收获重组包涵体蛋白,经变性、纯化、复性后获得重组鸡α干扰素。SDS-PAGE结果分析显示,目的蛋白可在原核表达载体中高效表达,发酵液上清中目的蛋白浓度高达0.2 mg/mL,分子量约为20 kDa。将鸡α干扰素稀释至活性为2.5×104 U/羽份,与灭活H9N2亚型流感病毒联用以口服的方式免疫无特定病原(SPF)鸡群,试验结果表明,短期(96 h)重复免疫3次,鸡α干扰素具有较好的安全性,可诱导鸡群的外周血、脾脏及胸腺产生较高水平的抗病毒相关的诱导基因,攻毒结果显示鸡α干扰素使用次数为连续使用3–5 d鸡群排毒率最低,体现出较好的抗流感病毒能力。本研究结果获得了鸡α干扰素的最佳免疫频率及免疫时间,为干扰素的最佳临床应用方法提供理论支撑。
关键词:鸡α干扰素H9N2亚型流感病毒口服短期重复
Inhibition of the replication of H9N2 influenza virus in vivo by short-term repeated oral administration of chicken interferon α
Meng Wang1,2, Jie Song1,2, Wenhui Fan1, Lirong Liu1,2, Zhuoran Huang3, Chengcheng Yang3, Hao Wu3, Wenjun Liu1,2

 , Jing Li1,2
, Jing Li1,2

1. Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China;
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Beijing Haidian Foreign Language Experiment School, Beijing 100195, China
Received: November 28, 2018; Accepted: March 19, 2019
Supported by: National Key Research and Development Program of China (No. 2017YFD051105), Youth Innovation Promotion Association of CAS (No. 2019091)
Corresponding author: Wenjun Liu. Tel: +86-10-64807497; Fax: +86-10-64807503; E-mail: liuwj@im.ac.cn;
Jing Li. Tel/Fax: +86-10-64807503; E-mail: lj418@im.ac.cn.
Abstract: To evaluate the optimal administration frequency for interferon-α (IFN-α) and the effect of its combined use with inactive virus on chicken flocks, the prokaryotic expression plasmid pET-22b-ChIFN-α was constructed and transferred into Escherichia coli BL21(DE3) host bacteria to induce the expression of chicken IFN-α and to harvest recombinant proteins inclusion bodies. The expression of recombinant chicken IFN-α was confirmed by SDS-PAGE, and the results demonstrated that the chicken IFN-α (20 kDa) was highly expressed using the prokaryotic expression vector with a concentration of 0.2 mg/mL in the medium. Chicken IFN-α was diluted to 2.5×104 U/fowls and administered to immunized specific-pathogen-free chickens orally in combination with inactivated H9N2 subtype influenza virus. Chicken that received chicken IFN-α were safe after three repeated immunizations (96 h). In addition, chicken IFN-α could induce higher levels of antiviral-related inducible genes in peripheral blood, spleen, and thymus of chicken flocks. The results of a challenge assay revealed that the lowest detoxification rates of chicken IFN-α ranged from three to five days, suggesting a higher capacity to resist H9N2 subtype avian influenza virus. The present study obtained the optimal immune frequency and immunization period for chicken IFN-α to provide theoretical support for the optimal clinical application of IFN-α.
Keywords: chicken interferon αH9N2 influenza virusesoral administrationshort-term repeated
干扰素(Interferon,IFN)是1957年英国****Isaacs和Lindenmann利用鸡胚绒毛尿囊膜研究流感病毒干扰现象时发现的[1]。它是一类重要的细胞因子,是由机体细胞受到病毒或其他诱生剂的作用而产生的一种分泌性糖蛋白,具有抑制细胞分裂、调节免疫、抗病毒、抗肿瘤等多种生物学活性[2],是生物体防御系统的重要组成部分。随着我国养禽业的快速发展,各种禽类疫病的发生也逐渐增多,特别是禽流感的流行给养禽业造成了严重的经济损失。禽流感是由A型流感病毒引起的一种禽类传染病,禽流感亚型众多,其中H9亚型流感病毒感染较为普遍[3],多表现为亚临床感染,引起呼吸系统疾病、产蛋率下降或急性致死,当前主要应用疫苗预防该类疾病,由于流感毒株的变异,免疫失败现象时有发生。随着抗病毒化学药品的禁用,将干扰素用于禽流感等病毒性疾病的治疗药物成为研究热点。
与哺乳动物相似,大多数禽类基因组中亦发现3种干扰素基因有可注释的基因组。鸡的Ⅰ型干扰素(IFN-α和IFN-β)具有独特的血清型,内含子较少,酸和热稳定。与哺乳动物的干扰素不同,鸡的IFN-α与哺乳动物的IFN-α全长相比只有24%的同源性,并且鸡Ⅰ型干扰素编码于Z性染色体上[4],Ⅰ型干扰素可被鸡新城疫等病毒诱导大量产生。鸡的Ⅱ型干扰素只有一个成员,即IFN-γ,调节免疫细胞的分化、成熟以及活性。不同于人有3种干扰素λ基因,鸡只有一种Ⅲ型干扰素基因[5-6],鸡的Ⅲ型干扰素目前发现与人类IFN-λ2具有相同的保守功能区,在上皮细胞和富含上皮的器官中表达量较高,其功能主要依赖于它的受体。
畜禽免疫的方式有多种,干扰素在临床应用常分为口服和注射方式,口服给药是一种较为便捷的投药方式,口服给药可以降低成本、副作用以及注射针头可能带来的血液传染病的风险。并且畜禽的口腔、咽部及肠道的黏膜血管丰富,口腔黏膜上存在模式识别受体[7],可以识别结合干扰素α,从而刺激口腔黏膜免疫发挥作用。通过黏膜表面传递疫苗抗原会激活黏膜B细胞和T细胞,刺激sIgA抗体反应,有助于产生远距离的黏膜免疫应答。同时鸡的IFN-α耐酸,pH 2.0–10.0都很稳定,能有效诱导黏膜系统的特异性免疫力,干扰素在食道及胃肠道的生物学活性仍然存在,特别适合大规模养殖场,也可避免注射给药带来的应激反应。近年来,较多研究侧重于干扰素诱导表达及纯化方法的构建以及其活性的检测,而探究如何高效地使用干扰素鸡群以更好地抵抗流感病毒以及如何正确将干扰素用于临床应用的报道并不多见。
为研究口服鸡α干扰素最佳的给药频率及与灭活疫苗联合使用对鸡群的影响,本研究将构建的原核表达质粒pET-22b-ChIFN-α,转入大肠杆菌Escherichia coli BL21 (DE3)宿主菌经诱导表达收获重组包涵体蛋白,经变性、纯化、复性后获得重组鸡α干扰素,将鸡α干扰素稀释至活性为2.5×104 U/羽份,与灭活H9N2亚型流感病毒共同以口服的方式免疫SPF鸡群,以期获得最佳免疫频率及最佳免疫时间,旨在为干扰素临床应用的正确使用方法提供理论支撑。
1 材料与方法1.1 质粒、菌、毒株及实验动物干扰素表达质粒pET-22b-ChIFN-α由本实验室构建;大肠杆菌TOP10、BL21 (DE3)由本实验室保存;鸡成纤维细胞(DF-1)、犬肾细胞(MDCK)由本实验室保存;SPF鸡胚(8日龄)购自北京维通利华实验动物技术有限公司;SPF白羽鸡(7日龄、8日龄)购自济南斯派福瑞禽业科技有限公司。H9亚型流感病毒A/Chicken/Taian/ 2013 (H9N2)株(TA株)由青岛蔚蓝生物制品有限公司分离、鉴定及保存。
1.2 主要试剂与设备Trizol Reagent购自美国赛默飞世尔科技公司;逆转录酶M-MLV、RNA酶抑制剂购自Promega公司;dNTPs、Oligo (dT)和SYBR Premix Ex TaqTM kit购自大连宝生物有限公司;蛋白胨、酵母粉购自北京华博德亿生物技术有限公司;蛋白预染Marker购自宝林科(北京)生物科技有限公司;异丙基-β-D-硫代半乳糖苷(IPTG)购自Promega。Scientz-ⅡD超声波细胞破碎仪购自宁波新芝生物科技股份有限公司;AKTA purifier UPC10为美国GE公司生产;倒置显微镜产自重庆光电仪器有限公司;CO2培养箱产自日本三洋电机公司;HSP-160生化培养箱产自北京东联哈尔仪器制造有限公司;7500实时定量PCR仪产自美国应用生物系统公司(ABI);D1000高效组织细胞破碎仪购自湖北新纵科病毒疾病工程技术有限公司。本试验所有引物由北京博迈德基因技术有限公司合成。
1.3 鸡α干扰素的制备及活性测定将重组质粒pET-22b-ChIFN-α转化到大肠杆菌E. coli BL21 (DE3)中,涂平板,37 ℃过夜,挑取平板上长出的单菌落于5 mL按1︰1 000加入50 μg/mL氨苄(Amp+)抗性的LB培养基中,将试管置于摇床上37 ℃、200 r/min培养至OD600值达0.5后,按1︰1 000加入1 mmol/L的IPTG诱导4 h。收集菌体,超声(400 W,超声3 s,间歇4 s)破菌后进行SDS-PAGE检测目的蛋白表达情况,重组蛋白以包涵体形式存在。依次用PBS、2 mol/L尿素和1 mol/L NaCl溶液将表达产物各洗涤2遍,随后加入6 mol/L盐酸胍室温下变性5 h至溶解,12 000 r/min离心15 min,分离出上清,加入3 mL的Ni-NTA agarose,旋转仪上4 ℃旋转结合4 h。以200 mmol/L咪唑洗脱目的蛋白,至考马斯亮蓝G-250蛋白检测液不再变色。按1︰40将目的蛋白洗脱液逐滴加入复性液,确保整个过程中没有沉淀析出,随后静置48 h。稀释复性后的ChIFN-α经0.22 μm滤器过滤除菌,4 ℃保存,利用细胞病变抑制法测定重组鸡α干扰素活性。
1.4 鸡α干扰素口服疫苗的制备测定H9N2病毒的血凝效价,无菌条件下用PBS溶液进行稀释,若血凝效价≥26,按1︰1 000稀释,若血凝效价 < 26,则按1:100稀释备用。取稀释过的病毒200 μL注入SPF鸡胚中,石蜡封口,将鸡胚继续放入培养箱中,37 ℃孵育3 d,每天翻转2次,舍去24 h内死亡的鸡胚。待鸡胚孵育72 h后,取出置于4 ℃冰箱冷藏过夜,收获鸡胚尿囊液并进行无菌检验。按照1︰1 000的比例往尿囊液中加入经过0.22 μm微孔滤膜过滤过的甲醛溶液,随后置于37 ℃摇床中50 r/min振荡过夜,并根据血凝试验检测病毒血凝效价。取灭活后的病毒液加入刚传代且状态良好的MDCK细胞中培养,盲传两代后,取上层培养基进行血凝试验检测是否灭活完全。将鸡α干扰素与灭活完全的病毒液混合,按照1︰1的比例制成口服候选疫苗LW001 (含2.5×104 U/mL),用于口服免疫。同时设立灭活完全的病毒液阳性对照及PBS阴性对照组。
1.5 动物安全性试验每组选取1日龄和7日龄雏鸡各10羽、5周龄雌性BALB/c小鼠10只、4月龄健康的雌性日本大耳白种兔6只,分别口服候选疫苗(LW001) 0.3 mL和2 mL,观察14 d,记录局部反应和临床症状。
1.6 SPF鸡免疫试验将SPF鸡随机分为4组,每组26羽,试验分组见表 1,每羽鸡免疫剂量为0.3 mL/只,口腔灌入,每隔48 h口服免疫一次。
表 1 SPF鸡分组免疫情况Table 1 Immunization regimens of oral vaccine
| Group ID | Candidate vaccine | Immune interval (d) | Immune cycle |
| 1 | LW001 (2.5×104U/ 0.1 mL) | 2 | 5 |
| 2 | Inactivated venom (105.0 EID 50/0.1mL) | 2 | 5 |
| 3 | ChIFN-α | 2 | 5 |
| 4 | PBS (Control) | 2 | 5 |
表选项
分别在0 h、48 h、96 h及240 h每组随机挑选4只SPF鸡进行采样,取外周血、脾脏、气管及胸腺,剪取0.1 mg的组织块,加入1 mL Trizol试剂,利用组织细胞破碎仪磨碎后放至离心管中研磨,使其充分裂解,?80 ℃保存备用。取外周血时按1︰1的比例加入抗凝剂后立即混匀,在离心管中按照1︰3的体积比加入Trizol试剂,总体积为1 mL;免疫时间点、免疫次数及采样时间点见图 1。
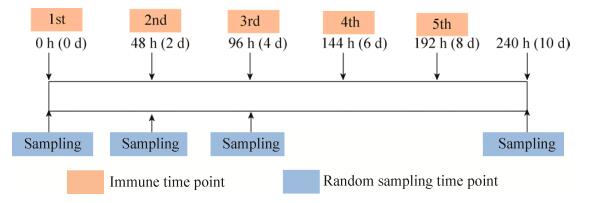 |
| 图 1 免疫及采样的试验方案图 Fig. 1 Immunization and sampling techniques. |
| 图选项 |
1.7 荧光定量PCR测定体内干扰素诱导基因水平将各时间段收获样品的总RNA用Oligo(dT)引物进行反转录获得cDNA,qPCR检测IL-2、IL-12、CCR10、IFN-β、MHC-Ⅰ和GAPDH的mRNA水平,分别用表 2中的引物进行扩增,以内参基因GAPDH作为对照。qPCR反应程序为:95 ℃预变性30 s;95 ℃ 5 s,60 ℃ 30 s,40个循环;60 ℃收集荧光。应用内参基因对校准样本和待测样本进行校正,进行相对定量检测。
表 2 qPCR引物序列表Table 2 Primer sequences used for qPCR
| Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
| IL-2 | CTTTGGCTGTATTTCGG | CTGGGTCTCAGTTGGTGT |
| IL-12 | RCATTTGCCCATTGGAGTCTAC | AGATGCTGGCAACTACACCTG |
| CCR10 | ACGTCTGCCTGCTGCACCT | CGCTGTAGAAGTTGAGGGC |
| IFN-β | CCTCTTCAACATGCTTAGCAGCC | TGTTGATGCTGAGGTGAGCGTTG |
| MHC-I | ACAAGTACCAGTGCCGCGTGGAGC | ACGATGGGCACCAGGTTGGGCTGT |
| GAPDH | TGCCATCACAGCCACACAGAAG | ACTTTCCCCACAGCCTTAGCAG |
表选项
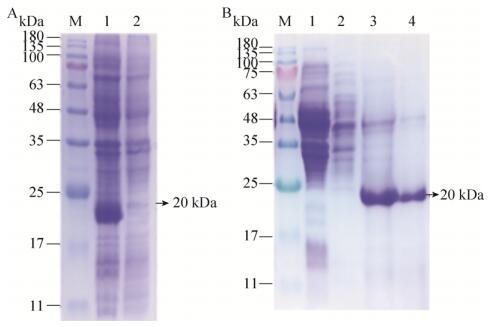 |
| 图 2 鸡α干扰素表达及纯化结果 Fig. 2 Expression and purification of recombinant chicken interferon-α protein. (A) M: protein marker; lane 1: expressed product after IPTG induction; lane 2: expressed product before IPTG induction. (B) M: protein marker; lane 1: expressed products after IPTG induction; lane 2: the supernatant after lysis; lane 3: the supernatant before purification; lane 4: purified recombinant protein. |
| 图选项 |
1.8 攻毒检测SPF鸡排毒率口服给药14 d后攻毒,TA株病毒液翅静脉注射0.2 mL/只(105.0 EID50/0.1mL),于攻毒第3、5、7日采集喉头与泄殖腔棉拭子,处理后分别尿囊腔接种10–11日龄SPF鸡胚5枚,0.2 mL/胚,孵育观察5 d,逐胚测定鸡胚液红细胞凝集价,每个拭子样品接种的5枚鸡胚中,有1枚鸡胚液的红细胞凝集价不低于1︰16 (微量法),即判为病毒分离阳性,对病毒分离阴性的样品,盲传1次后再判定,计算排毒率。使用SPSS卡方检验对阳性率进行统计学分析,P < 0.01差异极显著,P < 0.05差异显著。
2 结果与分析2.1 鸡α干扰素表达纯化及活性测定通过SDS-PAGE、考马斯亮蓝染色、脱色液脱色后,可以发现目的蛋白(20 kDa)在IPTG诱导下成功表达(图 1A)。通过包涵体洗涤、分子筛纯化后进行SDS-PAGE、考马斯亮蓝染色、脱色液脱色,结果如图 1B所示,鸡α干扰素表达及纯化结果较好。检测活性结果显示,根据在DF-1 (鸡成纤维细胞)上利用细胞病变抑制法检测,利用Reed-Muench法计算,鸡α干扰素的活性为106.5 U/0.1 mL,即107.5 U/mL,将其稀释至2.5×104 U/mL。
2.2 动物安全性检测试验为了检测鸡α干扰素疫苗实验室制品的安全性,分别给健康的雏鸡、BALB/c小鼠及日本大耳白种兔接种候选疫苗,结果显示,免疫的动物精神状态、饮水、采食、粪便、注射局部等均无异常,无流感的临床症状(表 3)。结果表明鸡α干扰素以口服方式免疫实验动物均是安全的。
表 3 鸡α干扰素的动物安全性检测Table 3 The safety of different batches of chicken interferon α vaccines
| Species | Batches | Challenge does (mL) | Number | Method of administration | Clinical signs |
| Chickens | LW001 | 0.3 | 5 | Oral administration | - |
| PBS | 0.3 | 5 | Oral administration | - | |
| LW001 | 2.0 | 5 | Oral administration | - | |
| PBS | 2.0 | 5 | Oral administration | - | |
| Rabbits | LW001 | 0.3 | 3 | Oral administration | - |
| PBS | 0.3 | 3 | Oral administration | - | |
| LW001 | 2.0 | 3 | Oral administration | - | |
| PBS | 2.0 | 3 | Oral administration | - | |
| BALB/c mice | LW001 | 0.3 | 5 | Oral administration | - |
| PBS | 0.3 | 5 | Oral administration | - | |
| LW001 | 2.0 | 5 | Oral administration | - | |
| PBS | 2.0 | 5 | Oral administration | - | |
| Clinical signs (?): alive without clinical symptoms. | |||||
表选项
2.3 口服鸡α干扰素对鸡群组织的影响取8日龄的SPF鸡,对鸡群免疫5次,免疫间隔为48 h,并于0、48、96、240 h采集外周血、脾脏、胸腺,利用荧光定量PCR的方法检测IL2、IL12、IFN-γ、MHC-Ⅰ、CCR10细胞因子的表达水平。
2.3.1 口服鸡α干扰素对外周血的影响鸡外周血中干扰素及粘膜免疫相关细胞因子mRNA的表达水平如图 3所示。在免疫干扰素和灭活病毒后48 h (第2次口服免疫后),外周血中的细胞因子IL2、IL12、IFN-γ、CCR10及MHC-Ⅰ的mRNA表达均开始有上升趋势,在96 h (第3次口服免疫后),上升趋势达到高峰,在240 h时外周血中的细胞因子略微下降。结果显示在96 h第3次口服干扰素后,与对照组相比,联合使用干扰素与灭活病毒的试验组(LW001),IL2、IL12、IFN-γ及CCR10的mRNA的表达水平较单独使用灭活病毒或单独使用干扰素的mRNA表达水平高。各因子进行统计学分析得出,LW001组IL2的表达量与ChIFN-α组相比较,P < 0.05,两组结果差异显著;LW001组CCR10的表达量与ChIFN-α组相比较,P < 0.01,两组在CCR细胞因子的mRNA表达水平上差异极显著。结果说明短期多次联合口服干扰素及灭活病毒,与抗病毒及天然免疫相关的细胞因子的表达水平较单独给药的对照组要高。
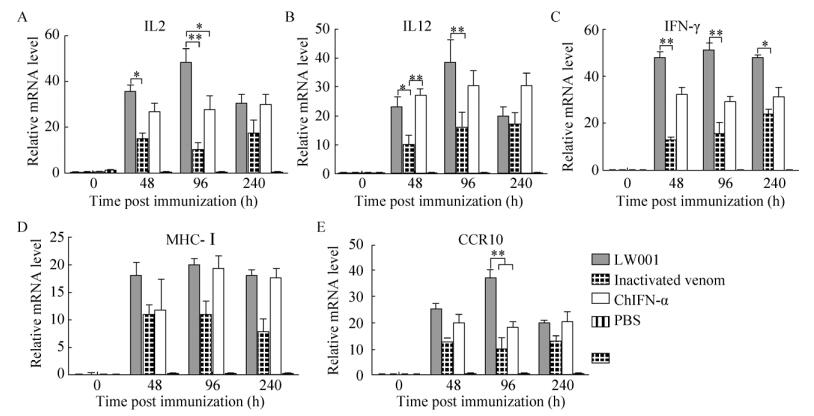 |
| 图 3 短期口服干扰素外周血中细胞因子mRNA水平 Fig. 3 The expression of mRNA in blood. (A) At 0, 48, 96, and 240 hpi, cells were harvested and extracted total RNA for quantitative real-time PCR assays. The mRNA levels of IL2 gene were detected by quantitative RT-PCR. (B) The mRNA levels of IL12 gene were detected by quantitative RT-PCR. (C) The mRNA levels of IFN-γ gene were detected by quantitative RT-PCR. (D) The mRNA levels of CCR10 gene were detected by quantitative RT-PCR. (E) The mRNA levels of MHC-Ⅰ gene were detected by quantitative RT-PCR. The β-actin mRNA served as an internal control. All date was shown as mean standard error of mean from three independent experiments. The relative mRNA level of genes treatments were compared with that of control at the same time points by the Mann-Whitney U test. Statistically significant differences are indicated (*P < 0.05, **P < 0.01). The asterisks were masked on the top of higher columns. |
| 图选项 |
2.3.2 口服鸡α干扰素对胸腺的影响鸡胸腺中干扰素及粘膜免疫相关细胞因子mRNA的表达水平如图 4所示。在短期多次口服干扰素的试验中,与对照组相比,第一次口服鸡α干扰素疫苗后的第48 h时,只有IL12和IFN-γ细胞因子的mRNA表达水平上调,其他上调不明显;第2次口服,在第96 h时,除IL-2和CCR10外,其余细胞因子mRNA水平均上调,LW001组中IFN-γ、MHC的mRNA表达水平与灭活病毒组相比差异极显著(P < 0.01),LW001组IL12的表达量与灭活病毒组相比差异显著(P < 0.05);在240 h时,细胞因子的mRNA表达量均有下降趋势。结果得出,在较长期口服干扰素的试验中,4 d时相比对照组,口服鸡α干扰素疫苗,mRNA表达水平变化均有明显的提高,持续2 d后开始下降。
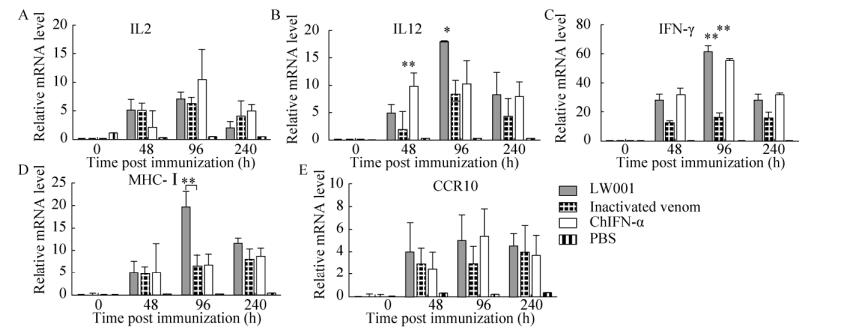 |
| 图 4 短期口服干扰素胸腺中细胞因子mRNA水平 Fig. 4 The expression of mRNA in thymus. (A) At 0, 48, 96, and 240 hpi, cells were harvested and extracted total RNA for quantitative real-time PCR assays. The mRNA levels of IL2 gene were detected by quantitative RT-PCR. (B) The mRNA levels of IL12 gene were detected by quantitative RT-PCR. (C) The mRNA levels of IFN-γ gene were detected by quantitative RT-PCR. (D) The mRNA levels of CCR10 gene were detected by quantitative RT-PCR. (E) The mRNA levels of MHC-Ⅰ gene were detected by quantitative RT-PCR. The β-actin mRNA served as an internal control. All date was shown as mean standard error of mean from three independent experiments. The relative mRNA level of genes treatments were compared with that of control at the same time points by the Mann-Whitney U test. Statistically significant differences are indicated (*P < 0.05, **P < 0.01). The asterisks were masked on the top of higher columns. |
| 图选项 |
2.3.3 口服鸡α干扰素对脾脏的影响鸡脾脏中干扰素及粘膜免疫相关细胞因子mRNA的表达水平如图 5所示,脾脏的结果与外周血的结果相似。在短期重复口服干扰素的试验中,与对照组相比,第一次口服鸡α干扰素疫苗后,所有检测的细胞因子mRNA水平在48 h表达量均上升;第2次口服鸡α干扰素疫苗后,在96 h处,疫苗组的IL2、IL12、IFN-γ、MHC-I的mRNA的表达呈现显著性上升水平;较长期口服鸡α干扰素疫苗后,在第10天采样检测后得出,除IFN-γ仍持续保持高水平外,其余各组mRNA表达量均有下调趋势。
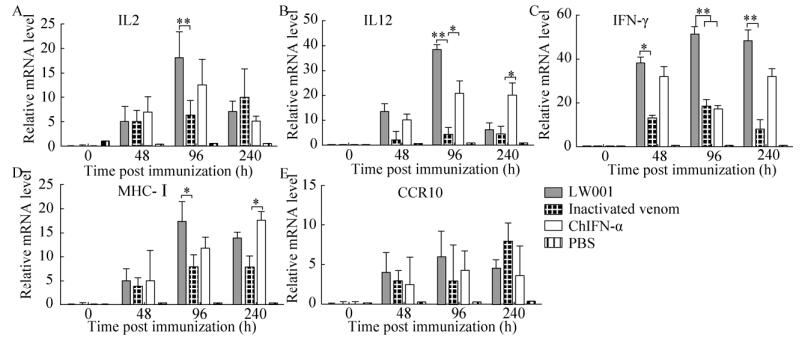 |
| 图 5 短期口服干扰素脾脏中细胞因子mRNA水平 Fig. 5 The expression of mRNA in spleen. (A) At 0, 48, 96, and 240 hpi, cells were harvested and extracted total RNA for quantitative real-time PCR assays. The mRNA levels of IL2 gene were detected by quantitative RT-PCR. (B) The mRNA levels of IL12 gene were detected by quantitative RT-PCR. (C) The mRNA levels of IFN-γ gene were detected by quantitative RT-PCR. (D) The mRNA levels of CCR10 gene were detected by quantitative RT-PCR. (E) The mRNA levels of MHC-Ⅰ gene were detected by quantitative RT-PCR. The β-actin mRNA served as an internal control. All date was shown as mean standard error of mean from three independent experiments. The relative mRNA level of genes treatments were compared with that of control at the same time points by the Mann-Whitney U test. Statistically significant differences are indicated (*P < 0.05, **P < 0.01). The asterisks were masked on the top of higher columns. |
| 图选项 |
2.4 攻毒后鸡群排毒率检测免疫鸡群4 d之后,使用H9亚型流感病毒进行攻毒试验,于攻毒后的3、5、7 d采集拭子,在鸡胚上利用EID50检测其排毒率,攻毒结果见表 4。
表 4 攻毒后鸡群排毒率检测情况Table 4 Detection of amount of IAV in chickens by challenge
| Group ID | Candidate vaccine | Immune interval (h) | By challenge with IAV | ||
| 3 d | 5 d | 7 d | |||
| 1 | LW001 (2.5×104 U/0.1 mL) | 48 | 6/10 | 5/10 | 6/10 |
| 96 | 5/10 | 2/10 | 2/10 | ||
| 240 | 6/10 | 6/10 | 5/10 | ||
| 2 | Inactivated venom (105.0 EID 50/0.1mL) | 48 | 8/10 | 8/10 | 8/10 |
| 96 | 8/10 | 7/10 | 7/10 | ||
| 240 | 8/10 | 9/10 | 8/10 | ||
| 3 | ChIFN-α | 48 | 6/10 | 6/10 | 6/10 |
| 96 | 5/10 | 5/10 | 3/10 | ||
| 240 | 5/10 | 4/10 | 4/10 | ||
| 4 | PBS (Control) | 48 | 10/10 | 10/10 | 10/10 |
| 96 | 10/10 | 10/10 | 10/10 | ||
| 240 | 10/10 | 10/10 | 10/10 | ||
表选项
检测其排毒量得出,短期重复使用鸡α干扰素疫苗组(96 h),在攻毒第3日排毒率为5/10,与攻毒对照组相比差异显著(P < 0.05),在攻毒第5日排毒率为2/10,鸡群排毒率呈下降趋势,与攻毒对照组相比差异极显著(P < 0.01),在攻毒第7日排毒率为2/10,鸡群排毒率仍保持较低比例(P < 0.01)。单独使用灭活病毒组,效果不理想,与PBS组差异不显著;单独使用鸡α干扰素组,排毒率最低的时间点也出现在96 h,显示短期重复使用鸡α干扰素也确实起到了抗病毒的作用,但效果没有与灭活病毒联用明显。综上LW001在连续使用96 h已达到保护效果,且使用效果优于其他对照组,因此为确保临床使用效果,将LW001使用次数定为连续使用3–5 d。
3 讨论在动物的免疫调节中,细胞免疫与体液免疫无疑是最重要的。干扰素影响细胞免疫与体液免疫的同时其在免疫紊乱中也有一定的作用[8]。目前,干扰素α是首个批准的治疗慢性乙型肝炎的药物,干扰素α已被证明在促进肝内CD8-T淋巴细胞反应与全身Th1免疫反应方面有重要的作用[9]。其中,与细胞免疫相关的细胞因子有IL-2、IL-12、IFN-γ等,与体液免疫相关的细胞因子包括IL-4、IL-6、IL-12等。另外,CCR10则是对淋巴细胞归巢中起作用的趋化因子受体,它们对淋巴细胞归巢以及相应的记忆/效应淋巴细胞的分化起着重要作用。CCR10在各种类型的细胞中表达,如T细胞、黑素细胞和皮肤内皮细胞以及血液中的IgA型浆细胞。有研究表明将分化后的DC细胞暴露于Ⅰ型干扰素会诱导其MHC-Ⅰ、MHC-Ⅱ及共刺激因子的表达[10-12]。也有报道发现干扰素α可以促进人DC细胞的分化与活化[13-14],成熟的DC细胞可以产生促炎症因子,如IL12等。Frydecka等[15]研究结果显示干扰素α是促炎症因子的潜在诱导剂,包括IL6。干扰素α通过JAK-STAT通路使接收干扰素信号的细胞内形成ISGF3复合物,该复合物诱导多种抗病毒基因的表达,如OAS、PKR等,并且有研究表明,鸡干扰素α在细胞水平有较好的抗病毒作用[16],还有研究人员比较了各型干扰素的细胞活性,比较发现干扰素α的抗病毒活性最高[17]。文中侧重于鸡干扰素α作为免疫佐剂,作用于鸡群的应用评价,采用短期重复的方案进行免疫鸡群,检测鸡干扰素α与灭活毒疫苗联用对鸡的免疫调节作用及抗病毒作用。检测了细胞因子在鸡α干扰素灭活毒疫苗给药后的表达情况,结果显示与细胞免疫及与体液免疫紧密相关的细胞因子均显著上升,说明口服鸡α干扰素灭活毒疫苗可以调节鸡的免疫水平,与先前文献报道的结论相符,并对先前研究从临床应用的角度加以补充。
据统计,约70%的病原体通过黏膜途径进入宿主体内[18]。IAV的复制主要在呼吸道上皮细胞中完成。黏膜抗原的传递可以增强局部和全身的免疫反应,这为免疫提供了一种有利的途径[19]。由于通过滴鼻的免疫途径,单独使用灭活病毒其免疫原性差[20-22],研究人员为了增强灭活病毒的免疫原性一般选取佐剂与其联用。目前针对H9N2亚型流感病毒灭活疫苗的佐剂选择有较多的研究,其中有以聚肌苷:聚胞二酸(poly I︰C)为佐剂的灭活疫苗,研究表明此疫苗可以快速提高抗体的水平并减少病毒滴度,其作用机制为上调Ⅰ型干扰素的产生并促进DC细胞的成熟与B细胞的活化,从而导致了CD4+T细胞与体液免疫应答[23-25]。文中以禽干扰素α作为佐剂与灭活病毒联用,产生了较好的机体黏膜免疫应答。
在禽类的体内脏器中,脾脏为免疫稳态的重要器官,是反映先天免疫与适应性免疫反应是否发挥作用的脏器[26]。胸腺也是机体重要的免疫器官,它与免疫耐受、免疫反应以及免疫活性T细胞的产生密切相关。试验结果表明鸡α干扰素与灭活H9N2亚型流感病毒联用在不同的免疫器官中可以引起不同的免疫调节,但是无论是外周血还是在脾脏和胸腺,均显示以鸡α干扰素为佐剂的灭活疫苗,较单独服用干扰素或灭活病毒有更好的免疫性及抗病毒效果。另外,短期重复口服鸡α干扰素灭活疫苗可以引起组织中免疫相关的细胞因子有规律的变化,细胞免疫密切相关IL2和IL12、传递抗原的重要分子MHC-Ⅰ[27]、巨噬细胞活化相关的IFN-γ[28]、黏膜免疫归巢因子CCR10都明显上调。这些mRNA的表达水平上调提示对应的蛋白会有可能上调,与抗病毒、免疫调节、细胞活化及免疫归巢因子相关的基因转录水平上调,对应的蛋白会增强机体的免疫水平同时起到抗病毒作用。试验数据显示组织中大多细胞因子于96 h表达量最高,这与攻毒试验中96 h时联合用药组排毒率最低的结果相符。当鸡吸入干扰素α时,IL2和IL12促炎性因子的表达量上升,促进了适应性免疫的活化,机体的免疫应答处于动态平衡,机体也随之产生抗炎机制以抵御炎症的出现[29],因此细胞因子会呈现先升高随后降低的规律性变化。
同时,脾脏是机体最大的血液过滤器[30],因此解释了本试验的研究数据,外周血与脾脏中细胞因子的表达量呈现相同变化趋势的结果。根据本研究试验数据,笔者建议家禽养殖场可通过每两天给鸡口服干扰素来增强鸡的免疫能力。
文中将纯化复性后的鸡α干扰素作为免疫佐剂与灭活H9N2亚型流感病毒联用,以口服的方式免疫SPF鸡群。短期重复口服鸡α干扰素后,鸡群的外周血、脾脏及胸腺等体内产生较高的抗病毒相关基因的诱导表达,攻毒结果显示鸡α干扰素使用次数为连续使用3–5 d鸡群排毒量最低,体现出较好的抗流感病毒能力。为了保证给药完全,干扰素在免疫前24 h,鸡群禁止饮水,免疫时按照100 mL/只的量计算出鸡群一天需要的总饮水量,在鸡场监控下保证鸡群2 h内饮完。但目前鸡α干扰素与病毒联用剂型确定、佐剂种类对比、与抗原混匀条件等方面的优化工艺还未完善,另外,稳定性试验、保存期试验、批间重复性试验等也将是后续工作的重点。
参考文献
| [1] | Santhakumar D, Rubbenstroth D, Martinez-Sobrido L, et al. Avian interferons and their antiviral effectors.Front Immunol, 2017, 8: 49. |
| [2] | Savan R, Ravichandran S, Collins JR, et al. Structural conservation of interferon gamma among vertebrates.Cytokine Growth Factor Rev, 2009, 20(2): 115–124.DOI: 10.1016/j.cytogfr.2009.02.006 |
| [3] | Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines.J Biol Chem, 2007, 282(28): 20059–20063.DOI: 10.1074/jbc.R700016200 |
| [4] | Goossens KE, Ward AC, Lowenthal JW, et al. Chicken interferons, their receptors and interferon-stimulated genes.Dev Comp Immunol, 2013, 41(3): 370–376.DOI: 10.1016/j.dci.2013.05.020 |
| [5] | Karpala AJ, Morris KR, Broadway MM, et al. Molecular cloning, expression, and characterization of chicken IFN-λ.J Interferon Cytokine Res, 2008, 28(6): 341–350.DOI: 10.1089/jir.2007.0117 |
| [6] | Feller L, Altini M, Khammissa RAG, et al. Oral mucosal immunity.Oral Surg Oral Med Oral Pathol Oral Radiol, 2013, 116(5): 576–583.DOI: 10.1016/j.oooo.2013.07.013 |
| [7] | SrivastavaA, GowdaDV, MadhunapantulaSV, 等. Mucosal vaccines: a paradigm shift in the development of mucosal adjuvants and delivery vehicles.APMIS, 2015, 123(4): 275-288. |
| [8] | Zdrojewicz Z, Pachura E, Pachura P. The thymus: a forgotten, but very important organ.Adv Clin Exp Med, 2016, 25(2): 369–375.DOI: 10.17219/acem/58802 |
| [9] | Zhang Z, Zhang HF, Chen DW, et al. Response to interferon-α treatment correlates with recovery of blood plasmacytoid dendritic cells in children with chronic hepatitis B.J Hepatol, 2007, 47(6): 751–759.DOI: 10.1016/j.jhep.2007.07.019 |
| [10] | Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type Ⅰ interferons.Nat Rev Immunol, 2015, 15(4): 231–242.DOI: 10.1038/nri3806 |
| [11] | Rouzaut A, Garasa S, Teijeira á, et al. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-α.Eur J Immunol, 2010, 40(11): 3054–3063.DOI: 10.1002/eji.201040523 |
| [12] | Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3β, and Th-1 chemokines in type Ⅰ IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities.Blood, 2001, 98(10): 3022–3029.DOI: 10.1182/blood.V98.10.3022 |
| [13] | Aricò E, Belardelli F. Interferon-α as antiviral and antitumor vaccine adjuvants: mechanisms of action and response signature.J Interferon Cytokine Res, 2012, 32(6): 235–247.DOI: 10.1089/jir.2011.0077 |
| [14] | Santini SM, Lapenta C, Logozzi M, et al. Type Ⅰ interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-Scid mice.J Exp Med, 2000, 191(10): 1777–1788.DOI: 10.1084/jem.191.10.1777 |
| [15] | Frydecka D, Paw?owski T, Pawlak D, et al. Functional polymorphism in the interleukin 6 (IL6) gene with respect to depression induced in the course of interferon-α and ribavirin treatment in chronic hepatitis patients.Arch Immunol Ther Exp (Warsz), 2016, 64(S1): 169–175.DOI: 10.1007/s00005-016-0441-7 |
| [16] | Dai MM, Wu SY, Feng M, et al. Recombinant chicken interferon-alpha inhibits the replication of exogenous avian Leukosis virus (ALV) in DF-1 cells.Mol Immunol, 2016, 76: 62–69.DOI: 10.1016/j.molimm.2016.06.012 |
| [17] | Qu HR, Yang LM, Meng SS, et al. The differential antiviral activities of chicken interferon α (ChIFN-α) and ChIFN-β are related to distinct interferon-stimulated gene expression.PLoS ONE, 2013, 8(3): e59307.DOI: 10.1371/journal.pone.0059307 |
| [18] | Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery.Annu Rev Biomed Eng, 2012, 14: 17–46.DOI: 10.1146/annurev-bioeng-071811-150054 |
| [19] | Kang SM, Guo LZ, Yao QZ, et al. Intranasal immunization with inactivated influenza virus enhances immune responses to coadministered simian-human immunodeficiency virus-like particle antigens.J Virol, 2004, 78(18): 9624–9632.DOI: 10.1128/JVI.78.18.9624-9632.2004 |
| [20] | Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen.Nat Immunol, 2001, 2(8): 725–731.DOI: 10.1038/90667 |
| [21] | Hagenaars N, Mastrobattista E, Glansbeek H, et al. Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model.Vaccine, 2008, 26(51): 6555–6563.DOI: 10.1016/j.vaccine.2008.09.057 |
| [22] | Tseng LP, Chiou CJ, Chen CC, et al. Effect of lipopolysaccharide on intranasal administration of liposomal Newcastle disease virus vaccine to SPF chickens.Vet Immunol Immunopathol, 2009, 131(3/4): 285–289. |
| [23] | Zhang AG, Lai HZ, Xu JH, et al. Evaluation of the protective efficacy of poly I:C as an adjuvant for H9N2 subtype avian influenza inactivated vaccine and its mechanism of action in ducks.PLoS ONE, 2017, 12(1): e0170681.DOI: 10.1371/journal.pone.0170681 |
| [24] | Swayne DE. Avian influenza vaccines and therapies for poultry.Comp Immunol Microbiol Infect Dis, 2009, 32(4): 351–363.DOI: 10.1016/j.cimid.2008.01.006 |
| [25] | Tang YH, Lu JH, Wu PP, et al. Inactivated vaccine with adjuvants consisting of pattern recognition receptor agonists confers protection against avian influenza viruses in chickens.Vet Microbiol, 2014, 172(1/2): 120–128. |
| [26] | Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers.Nat Immunol, 2012, 13(8): 722–728.DOI: 10.1038/ni.2366 |
| [27] | Gainey MD, Rivenbark JG, Cho H, et al. Viral MHC class I inhibition evades CD8+ T-cell effector responses in vivo but not CD8+ T-cell priming.Proc Natl Acad Sci USA, 2012, 109(47): E3260–E3267.DOI: 10.1073/pnas.1217111109 |
| [28] | Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-γ: an overview of signals, mechanisms and functions.J Leukoc Biol, 2004, 75(2): 163–189.DOI: 10.1189/jlb.0603252 |
| [29] | Chen Z, Bozec A, Ramming A, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis.Nat Rev Rheumatol, 2019, 15(1): 9–17. |
| [30] | Mebius RE, Kraal G. Structure and function of the spleen.Nat Rev Immunol, 2005, 5(8): 606–616.DOI: 10.1038/nri1669 |
