

中国医学科学院 北京协和医学院 血液学研究所 血液病医院 实验血液学国家重点实验室,天津 300020
收稿日期:2018-03-27;接收日期:2018-06-14 基金项目:国家自然科学基金(Nos.81629001, 81470280), 天津科学技术委员会青年基金(Nos.15JCQNJC12100, 17JCQNJC09800), 北京协和医学院“协和青年基金”(Nos.3332016141, 3332016092)资助
摘要:Tet2 (Tet家族成员2)在DNA去甲基化修饰、表观遗传调控及骨髓造血中起着重要作用。笔者课题组前期研究发现,随着年龄增长,Tet2敲除小鼠逐步发展为淋系白血病和髓系白血病。但Tet2在骨髓微环境中的作用仍不清楚。进一步研究发现,Tet2敲除的骨髓间充质干细胞(Mesenchymal stem cells,MSC)更多处于G2/M分裂期,其细胞分裂时间缩短,生长速度加快。长周期培养-起始细胞实验表明,Tet2敲除的MSC支持造血干细胞扩增和髓系分化的能力增强。通过点杂交实验发现,Tet2敲除后,骨髓细胞DNA总甲基化水平升高。对Tet2缺失的骨髓细胞进行甲基化测序,结果表明:基因组转录调控区域等多个功能性结构域的甲基化水平明显升高。同时,敲除Tet2的MSC分泌IL-8、IL-18等炎性细胞因子的能力下降;敲除Tet2的MSC更多分泌促进造血干细胞髓系分化的GM-CSF和CCL-3等细胞因子。Tet2可以影响间充质干细胞造血支持作用,进而调节造血。
关键词:Tet基因家族成员2表观遗传调控DNA甲基化间充质干细胞
Tet2 regulates the function of mesenchymal stem cells
Jie Gu, Yuxia Wang, Juan Gao, Shengnan Yuan, Yajing Chu, Yanhan Li, Weiping Yuan, Xiaomin Wang


State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Tianjin 300020, China
Received: March 27, 2018; Accepted: June 14, 2018
Supported by: National Natural Science Foundation of China (Nos. 81629001, 81470280), Tianjin Science and Technology Commission (Nos. 15JCQNJC12100, 17JCQNJC09800), Peking Union Medical College Youth Grant (Nos. 3332016141, 3332016092)
Corresponding author:Xiaomin Wang. Tel: +86-22-23909166; Fax: +86-22-23909418; E-mail: wangxiaomin@ihcams.ac.cn
Abstract: Tet2 (member 2 of the Tet family) plays an important role in DNA demethylation modification, epigenetic regulation, and hematopoiesis. In our previous study, we found that Tet2 knockout mice progressively developed lymphocytic leukemia and myeloid leukemia with aging. However, the role of Tet2 in bone marrow microenvironment is unclear. Here in this study, we found that more Tet2-/- mesenchymal stem cells (MSCs) from bone marrow were in the G2/M cell cycle stages. The division time of Tet2-/- MSCs was shorter than that of the control cells. The growth rate of Tet2-/- MSCs was accelerated. The cobblestone area-forming cells assay (CAFC) showed that Tet2 knockout MSCs supported the expansion of hematopoietic stem cells (HSCs) and the differentiation of HSCs was skewed towards myeloid cells. Through the dot blotting experiment, we found that the total methylation level was increased in Tet2-/- bone marrow cells (BM). We used the methylation-chip to analyze the methylation level of Tet2-/- bone marrow cells and found that the level of methylation was increased in the transcriptional starting area (TSS), exons (EXONS) and 3' untranslated region (3' UTR). Moreover, we found that the cytokines secreted by Tet2-/- MSCs, such as IL-8 and IL-18, were decreased. While the expressions of GM-CSF and CCL-3, which supported hematopoietic stem cells to differentiate to myeloid cells, were increased in Tet2-/- MSCs. Our results demonstrated that Tet2 regulates MSCs to support hematopoiesis.
Keywords: member 2 of the Tet familyepigenetic regulationDNA methylationmesenchymal stem cells
DNA的甲基化修饰是基因表达调控的重要环节,肿瘤细胞往往表现为典型的DNA高甲基化,尤其是胞嘧啶-磷酸-鸟嘌呤(CpG)启动子区域肿瘤抑制基因的高甲基化[1-2]。肿瘤抑制基因的高甲基化通过改变染色质状态,招募辅助活化因子或抑制因子,使基因转录活化受到抑制,导致肿瘤抑制基因表达沉默。TET蛋白属于依赖酮戊二酸和亚铁离子的双加氧酶,其催化5-甲基胞嘧啶(5-mC)转化为5-羟甲基胞嘧啶(5-hmC),并进一步转化为5-甲酰胞嘧啶(5-fC)和5-羧基胞嘧啶(5-caC),在胚胎发育和基因重新编码等过程中都起着重要作用[3-5]。对染色体4q24最小区域杂合性缺失和微缺失的筛查显示,骨髓增殖性肿瘤(Myeloproliferative neoplasms,MPN)和MDS (Myelodysplastic syndrome,MDS)患者中往往存在TET2缺失和失活性突变[6-7]。TET2突变在MPN和MDS中的发生率为10%?20%,在急性髓细胞白血病(Acute myeloid leukemia,AML)中为7%?23%[8]。5-hmC可阻止甲基化DNA与相关蛋白的结合,进而抑制其转录,产生沉默作用。对胚胎干细胞的研究表明,5-hmC富集在靠近转录起始点和基因内的CpG二核苷酸区,且5-hmC在这些调控区域的富集均与基因表达增强有关[9]。研究发现,在MDS和慢性粒单核细胞白血病(Chronic myelomonocytic leukaemia,CMML)中,TET2突变可发生在包括造血干细胞的CD34+细胞,且有些患者同时存在多个TET2突变点,提示TET2突变可导致早期干细胞获得克隆性生长优势[10-11]。
既往研究大多局限于造血细胞TET2异常导致的造血改变及其临床意义,而TET2是否具有调控MSC生物学特性及其造血支持能力的作用,目前仍不清楚。在本研究中,我们获取Tet2敲除小鼠(Tet2-/-)和正常小鼠(WT)骨髓MSC,通过比较研究,阐述Tet2基因调控MSC的生物学能力的作用及其可能机制。
1 材料与方法1.1 材料胎牛血清(Gibco公司),CCK-8 (DojinDo Laboratories公司),Spectra Max M5 Microplate Reader (Molecular Devices公司),细胞培养箱(Thermo公司)。
Tet2基因全身敲除小鼠购于The Jackson Laboratory (https://www.jax.org/strain/023359),货号为023359。
1.2 方法1.2.1 骨髓间充质干细胞分离和培养取Tet2-/-小鼠双下肢股骨和胫骨,冲出骨髓细胞,将骨髓细胞用2 mL HBSS缓冲液洗涤2遍(枪尖吹匀),1 500 r/min离心6 min,将骨髓细胞均匀平铺于10 cm培养皿中,10%胎牛血清IMDM培养1周。1周后可见间充质干细胞呈现克隆样生长。去除培养基,换新鲜培养基继续培养。每隔3–4 d待细胞长满后分瓶传代。
1.2.2 细胞增殖实验收集对数生长期细胞,计数,用完全培养基重新悬浮细胞,调整细胞浓度至合适浓度,接种96孔板,每孔加100 μL细胞悬液。细胞在37 ℃、100%相对湿度,5% CO2培养箱中培养1?7 d。每天取一个培养板,加入含10% CCK-8的完全培养基置于37 ℃培养箱中孵育3 h。轻轻振荡后在Spectra Max M5 Microplate Reader上测定450 nm波长处的吸光度。绘制生长曲线。
1.2.3 长周期培养-起始细胞实验于96孔板接种MSC,每组3个重复孔,每孔加100 μL培养基,在37 ℃、5% CO2孵箱中培养。待MSC融合率达到80%,照射20 Gray,在33 ℃、5% CO2孵箱中培养1 d。加入造血干细胞,每孔细胞数为500,培养基体积为每孔150 μL。加入Stem cell公司的5300长周期培养基150 μL。在33 ℃、5% CO2的孵箱中培养5周,每周半量换液。4周或5周后计数鹅卵石状克隆,细胞数大于5为一个克隆。
1.2.4 点杂交方法检测基因组甲基化水平提取细胞基因组DNA,超声破碎至合适大小。硝酸纤维素膜先用蒸馏水稍微浸湿,并用20×SSC使其饱和。将膜放在经20×SSC预饱和过的滤纸上。以5 μL的DNA分装样品在滤膜上点样。将滤膜放在一张干燥的滤纸晾干,以5×SSC漂洗5 min。将硝酸纤维素膜置80 ℃真空烤炉2 h。将DNA面用紫外线(280 mn)照射3?5 min,这时的滤膜已可用于杂交。预杂交液过夜封闭醋酸纤维素膜。加入anti-5-hmC或者anti-5-mC探针4 ℃过夜孵育。加入HRP结合的二抗1?2 h。化学显色。
1.2.5 全基因组DNA甲基化测序和数据分析利用hME-Seal方法从细胞中获得100?500 bp大小的DNA片段[12-13]。利用Illumina公司的甲基化芯片进行测序,并建立细胞甲基化数据库(NEBNext? ChIP-Seq Library Prep Reagent Set for Illumina)。数据解读参考2011年Szulwach和Yao等的研究方法[14-17]。
1.2.6 统计学分析按下式计算药物对肿瘤细胞生长的抑制率,并采用分析软件Graph Pad Prism 5.0拟合IC50曲线和计算IC50值。细胞生长抑制率(%)=[(Ac–As)/ (Ac–Ab)]×100%。As:样品的OA (细胞+CCK-8+待测化合物),Ac:阴性对照的OA (细胞+CCK-8+DMSO),Ab:阳性对照的OA (培养基+CCK-8+DMSO)。*P < 0.05,**P < 0.01,***P < 0.005,****P < 0.001。
2 结果与分析2.1 Tet2敲除的骨髓MSC生长状态良好取Tet2-/-小鼠四肢骨,获得骨髓细胞,培养骨髓细胞1周后,消化传代1次,继续培养1周,贴壁的细胞为骨髓来源的Tet2-/-MSC。我们检测了Tet2在Tet2-/- MSC和WT-MSC中的表达,发现在Tet2-/- MSC细胞中Tet2几乎不表达(图 1)。镜下观察Tet2-/- MSC形态,细胞形态均一,呈长梭形,扩增速度快,克隆形成能力强,体外培养1至2周可传代。传代后细胞生长速度明显加快,12 h内完全贴壁、伸展,重新变为长梭形,培养4?7 d即达到完全融合。在倒置显微镜下,细胞为梭形,胞浆丰富,核染色质细,核仁明显,呈平行排列或旋涡样生长(图 2)。
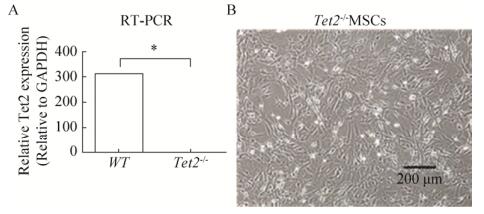 |
| 图 1 Tet2缺失的骨髓MSC Fig. 1 Tet2-/- MSC from BM. (A) Relative expression of Tet2 in Tet2-/- MSC. (B) Tet2-/- MSCs are spindle shaped, with rich cytoplasm, fine chromatin and obvious nucleolus. |
| 图选项 |
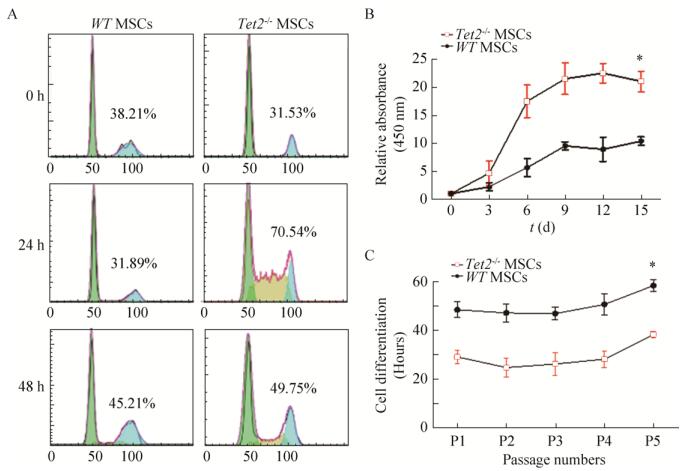 |
| 图 2 Tet2缺失导致MSC增殖能力的加快 Fig. 2 Tet2-/- deletion increased MSCs proliferation. (A) Cell cycle study with PI staining. (B) Cell growth curve. (C) The amplification time of MSCs with different passages. *P < 0.05. |
| 图选项 |
2.2 Tet2敲除的骨髓MSC细胞周期加快,细胞扩增时间缩短骨髓MSC在体外的增殖能力较强,但随着传代次数增加,其增殖速率及总体扩增倍数基本保持一致。我们前期研究发现,Tet2敲除可加速MSC生长。我们检测了Tet2-/- MSC细胞的生长周期,发现在血清刺激24 h和48 h后,Tet2-/- MSC处于S期的细胞比例明显高于WT-MSC (图 2A)。此外,根据细胞生长曲线(图 2B),Tet2-/- MSC细胞倍增时间(32.8 h±4.8 h)明显低于WT-MSC (49.4 h± 5.3 h),且随着传代次数增加,细胞倍增时间并不发生改变(图 2C)。上述结果提示Tet2-/- MSC增殖能力明显强于WT-MSC。
2.3 Tet2敲除的骨髓MSC在多次传代后仍然有很强的造血支持作用为了评估Tet2基因是否影响MSC支持造血的能力,骨髓造血祖细胞细胞被接种于照射过的Tet2-/- MSC和WT-MSC中,在长期骨髓培养基中培养4周后检测鹅卵石区形成细胞(Cobblestone area forming cell,CAFC)的生成情况。如图 3所示,Tet2-/- MSC和WT-MSC具有支持造血干细胞克隆形成能力(CAFC),但是Tet2-/- MSC支持CAFC的能力明显优于WT-MSC (P≤0.01) (图 3A, C, D)。将CAFC进行集落培养,培养一周后发现粒系集落生成单位(CFU-GM)在Tet2-/- MSC组的数量要明显高于WT MSC (P≤0.01) (图 3B)。MSC支持造血能力随着细胞传代并不发生改变。上述结果提示Tet2-/- MSC支持造血能力增强。
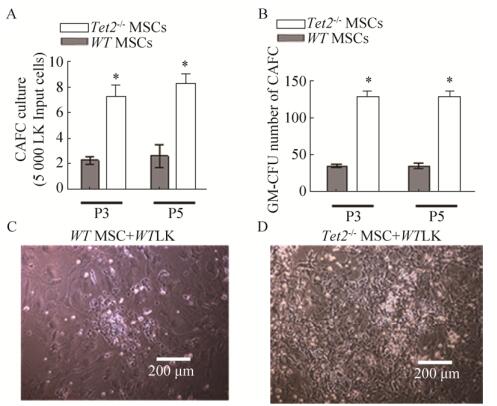 |
| 图 3 Tet2敲除导致MSC支持造血能力的增强 Fig. 3 Tet2-/- deletion enhances the ability of MSC to support LK cells proliferation. (A) CAFC assay was used to test the ability of Tet2-/- MSC to support LK cells proliferation. (B) CFC assay was used to test the differentiation of LK cells after treatment of Tet2-/- MSC. (C, D) Representative graphs of CAFC. * P < 0.05. |
| 图选项 |
2.4 Tet2敲除导致骨髓MSC甲基化水平增加为了检测Tet2基因是否影响MSC的甲基化状态,我们应用点杂交和甲基化测序检测Tet2-/- MSC和WT-MSC的甲基化状态。点杂交结果显示,同WT-MSC相比,Tet2-/- MSC全基因组中5mc明显增加,而5hmc明显减低(图 4A)。甲基化测序的结果进一步证实,Tet2-/- MSC全基因组中的5hmc数量明显低于WT-MSC (图 4B)。随后的分析发现异常改变的5hmc主要分布于转录起始区(TSS)、外显子区(EXONS)和3'非翻译区(3' UTR) (图 4C)。上述结果说明Tet2基因可以显著影响MSC全基因组的甲基化状态。
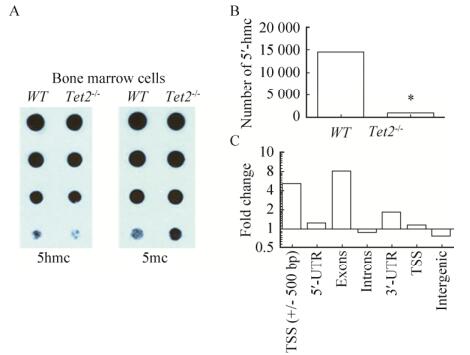 |
| 图 4 Tet2敲除导致骨髓细胞甲基化水平升高 Fig. 4 Tet2-/- deletion increases the methylation level of bone marrow (BM). (A) Genomic DNA was extracted from BM cells of WT or Tet2-/- mice and blotted onto nitrocellulose membrane after 2-fold serial dilution. 5hmC and 5mC levels were detected with an anti-5hmC (ActiveMotif, #39791) or 5mC (Calbiochem; NA#81) antibody. (B) Methylation-chip demonstrated that the methylation level was incresed in Tet2-/- BM cells. (C) Methylation level was incresed in the transcriptional starting area (TSS), exons (EXONS) and 3' untranslated region (3' UTR) of Tet2-/- BM cells. *P < 0.05. |
| 图选项 |
2.5 Tet2异常的骨髓MSC分泌造血调控因子功能的改变为了进一步明确Tet2-/- MSC支持造血能力改变的原因,我们通过细胞因子芯片方法发现,许多常见的由MSC分泌的细胞因子(包括黏附分子和造血调控因子)在Tet2-/- MSC的培养上清液中表达异常。其中,IL-16、IL-6、TREM1、TNF-α、sICAM1、CXCL2、G-CSF明显减低,而CCL5、GM-CSF、SDF1和CCL3明显增加(图 5)。上述结果提示Tet2基因影响MSC的关键细胞因子表达,进而改变其造血支持功能。
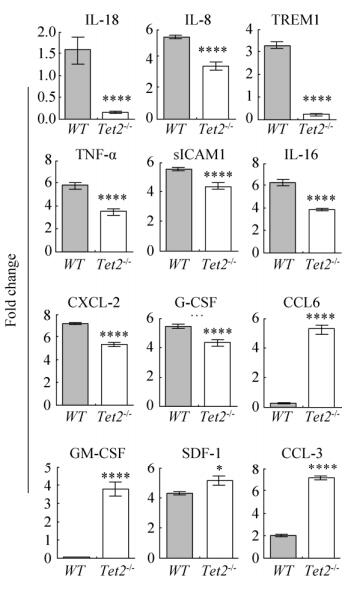 |
| 图 5 Tet2敲除骨髓MSC分泌细胞因子能力改变 Fig. 5 The cytokine secreted by Tet2-/- MSC is changed. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. |
| 图选项 |
3 讨论正常造血依赖于复杂而完整的骨髓造血微环境,骨髓微环境主要通过骨髓内细胞与细胞间的相互作用、基质细胞产生的生长或抑制因子以及细胞基质与造血细胞间的相互作用来调节造血功能[18-19]。MSC是一类具有自我更新和多向分化能力的多能干细胞。存在于骨髓中的MSC是骨髓基质细胞的前体细胞,可形成骨髓和外膜间质细胞,构成造血的微环境,MSC分化形成的结缔组织骨架及该骨架产生的细胞因子、化学因子和ECM可调节造血细胞的归巢和增殖;MSC还能够分泌多种造血因子,表达多种表面粘附因子,在造血调控的过程中发挥着重要作用[19]。
DNA甲基化是最常见的一种表观遗传学修饰,广泛参与干细胞增殖、分化和转化相关的关键基因群的表达调控。既往研究证实DNA甲基化在造血干细胞(Hematopoietic stem cell,HSC)增殖和分化中发挥重要调控作用[20-21]。但是,
DNA甲基化是否具有调控MSC生物学性状的能力,目前尚不清楚。TET2是目前发现最关键的DNA甲基化调控基因之一,不仅具有调控HSC的功能,还是关键的抑癌基因,在血液系统肿瘤的发生中发挥作用[22-23]。TET2是调控DNA羟甲基化最重要的胞嘧啶甲基化双氧酶,TET2能够将5mc转化成5hmc。Li等证实Tet2敲除的造血干祖细胞中5mc明显增加,而5hmc相应降低[21]。我们的研究发现:同WT-MSC比较,Tet2-/- MSC全基因组中5mc明显增加,而5hmc明显减低,提示Tet2基因可以显著影响MSC全基因组的甲基化状态。骨髓MSC具有很强的增殖能力,可以有效在体外扩增获得细胞治疗需求的细胞量。既往研究发现关键基因的变化可以影响MSC的自我更新和增殖能力。Zhang等的研究发现Asxl1敲除MSC的自我更新和体外增殖能力都明显下降[24]。本研究中,我们发现,同WT-MSC比较,Tet2-/- MSC增殖能力明显增加,这种改变的增殖能力随着细胞传代并不发生改变。上述结果提示Tet2基因的异常可以显著改变MSC的生物学性状。
作为骨髓微环境中最重要的细胞群,MSC通过和HSC的直接接触,激活和关闭调控HSC的诸多信号通路,发挥调控HSC的作用。此外,MSC还能合成多种造血调节因子,发挥维持长期培养起始细胞的增殖、支持造血和促进造血恢复的作用,由此可见骨髓MSC可以通过模拟骨髓微环境发挥调控造血的功能。诸多外在因素(包括HSC和药物)可以影响骨髓MSC造血支持作用,重塑骨髓造血微环境,实现生理或者病理性造血调控。但是,表观遗传学相关基因是否具有调控MSC造血支持能力,目前尚不清楚。我们的研究通过骨髓长期培养和克隆生成实验,阐明Tet2-/- MSC维持HSC能力明显强于WT-MSC。进一步分析上述培养体系中CFU-GM的生成情况,结果表明Tet2-/- MSC对定向祖细胞的增殖能力明显优于WT-MSC。我们的结果说明,TET2可以影响MSC对不同阶段造血干祖细胞增殖和分化能力的调节作用。
越来越多的研究证实MSC通过分泌多种细胞因子调控造血,促进骨髓来源的CFU-GM、CFU-E和CFU-GEMM的增殖。Zhang等发现Tet2可以结合在IL-6启动子区,Tet2缺失可以促进炎症反应中树突细胞和巨噬细胞IL-6的分泌[25]。Greenbaum等发现在MSC中敲除CXCL12可以导致骨髓HSC数量明显减低,并影响其造血重建和分化功能,提示MSC分泌的CXCL12在维持正常HSC造血功能方面发挥重要作用[26]。本研究通过细胞因子芯片方法证实多种细胞因子(包括黏附分子和造血调控因子)在Tet2-/- MSC中异常表达。其中,IL16、IL6、TREM1、TNF-α、sICAM1、CXCL2、G-CSF明显减低;而CCL5、GM-CSF、SDF1和CCL3明显增加。提示Tet2通过影响MSC分泌异常的细胞因子,进而发挥调控造血的功能。
综上所述,我们的研究显示Tet2可以影响骨髓MSC基因组的甲基化状态,增加MSC体外增殖能力。此外,Tet2影响MSC对不同阶段造血干祖细胞增殖和分化能力的调节作用,这种造血调控能力可能与细胞因子途径相关。
参考文献
| [1] | Ziller MJ, Gu HC, Müller F, et al. Charting a dynamic DNA methylation landscape of the human genome.Nature, 2013, 500(7463): 477–481.DOI: 10.1038/nature12433 |
| [2] | Robertson KD. DNA methylation and human disease.Nat Rev Genet, 2005, 6(8): 597–610. |
| [3] | Tahiliani M, Koh KP, Shen YH, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1.Science, 2009, 324(5929): 930–935.DOI: 10.1126/science.1170116 |
| [4] | Ito S, D'Alessio AC, Taranova OV, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification.Nature, 2010, 466(7310): 1129–1133.DOI: 10.1038/nature09303 |
| [5] | Guo JU, Su YJ, Zhong C, et al. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain.Cell, 2011, 145(3): 423–434. |
| [6] | Jankowska AM, Szpurka H, Tiu RV, et al. Loss of heterozygosity 4q24 and TET2 mutations associated with myelodysplastic/myeloproliferative neoplasms.Blood, 2009, 113(25): 6403–6410.DOI: 10.1182/blood-2009-02-205690 |
| [7] | Ko M, Huang Y, Jankowska AM, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2.Nature, 2010, 468(7325): 839–843.DOI: 10.1038/nature09586 |
| [8] | Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers.N Engl J Med, 2009, 360(22): 2289–2301.DOI: 10.1056/NEJMoa0810069 |
| [9] | Fouse SD, Shen Y, Pellegrini M, et al. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation.Cell Stem Cell, 2008, 2(2): 160–169.DOI: 10.1016/j.stem.2007.12.011 |
| [10] | Itzykson R, Kosmider O, Renneville A, et al. Clonal architecture of chronic myelomonocytic leukemias.Blood, 2013, 121(12): 2186–2198.DOI: 10.1182/blood-2012-06-440347 |
| [11] | Smith AE, Mohamedali AM, Kulasekararaj A, et al. Next-generation sequencing of the TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones with early origins, but indicates no definite prognostic value.Blood, 2010, 116(19): 3923–3932.DOI: 10.1182/blood-2010-03-274704 |
| [12] | Song ZQ, Zhang QS. Design, synthesis, and incorporation of fluorous 5-methylcytosines into oligonucleotides.J Org Chem, 2011, 76(24): 10263–10268.DOI: 10.1021/jo2015399 |
| [13] | Song ZJ, Zhou RB, Li D, et al. Detection of p16INK4a promoter methylation status in non-small cell lung cancer by a fluorescence polarization assay.Diagn Mol Pathol, 2011, 20(3): 138–142. |
| [14] | Szulwach KE, Li XK, Li YJ, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging.Nat Neurosci, 2011, 14(12): 1607–1616.DOI: 10.1038/nn.2959 |
| [15] | Szulwach KE, Li XK, Li YJ, et al. Integrating 5-hydroxymethylcytosine into the epigenomic landscape of human embryonic stem cells.PLoS Genet, 2011, 7(6): e1002154.DOI: 10.1371/journal.pgen.1002154 |
| [16] | Yao LS, Li YY, Du FX, et al. Histone H4 Lys 20 methyltransferase SET8 promotes androgen receptor-mediated transcription activation in prostate cancer.Biochem Biophys Res Commun, 2014, 450(1): 692–696.DOI: 10.1016/j.bbrc.2014.06.033 |
| [17] | Yao B, Lin L, Street RC, et al. Genome-wide alteration of 5-hydroxymethylcytosine in a mouse model of fragile X-associated tremor/ataxia syndrome.Hum Mol Genet, 2014, 23(4): 1095–1107.DOI: 10.1093/hmg/ddt504 |
| [18] | Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration.Nat Med, 2014, 20(8): 833–846.DOI: 10.1038/nm.3647 |
| [19] | Krause DS, Scadden DT, Preffer FI. The hematopoietic stem cell niche-home for friend and foe?.Cytometry B Clin Cytom, 2013, 84(1): 7–20. |
| [20] | Pan F, Wingo TS, Zhao ZG, et al. Tet2 loss leads to hypermutagenicity in haematopoietic stem/progenitor cells.Nat Commun, 2017, 8: 15102.DOI: 10.1038/ncomms15102 |
| [21] | Li Z, Cai XQ, Cai CL, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies.Blood, 2011, 118(17): 4509–4518.DOI: 10.1182/blood-2010-12-325241 |
| [22] | Zhao ZQ, Chen L, Dawlaty MM, et al. Combined loss of Tet1 and Tet2 promotes B cell, but not myeloid malignancies, in mice.Cell Rep, 2015, 13(8): 1692–1704.DOI: 10.1016/j.celrep.2015.10.037 |
| [23] | Zhao Z, Chen S, Zhu X, et al. The catalytic activity of TET2 is essential for its myeloid malignancy- suppressive function in hematopoietic stem/ progenitor cells.Leukemia, 2016, 30(8): 1784–1788.DOI: 10.1038/leu.2016.56 |
| [24] | Zhang P, Xing CH, Rhodes SD, et al. Loss of Asxl1 alters self-renewal and cell fate of bone marrow stromal cells, leading to bohring-opitz-like syndrome in mice.Stem Cell Rep, 2016, 6(6): 914–925.DOI: 10.1016/j.stemcr.2016.04.013 |
| [25] | Zhang Q, Zhao K, Shen QC, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6.Nature, 2015, 525(7569): 389–393.DOI: 10.1038/nature15252 |
| [26] | Greenbaum A, Hsu YMS, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance.Nature, 2013, 495(7440): 227–230.DOI: 10.1038/nature11926 |
