

1 西南大学 家蚕基因组生物学国家重点实验室,重庆 400715;
2 重庆师范大学 生命科学学院,重庆 400047
收稿日期:2017-12-04;接收日期:2018-04-25 基金项目:国家自然科学基金(Nos. 31402138, 31601678),重庆市基础科学与前沿探索项目(No.cstc2018jcyjAX0550),中央高校基本科研业务费(Nos. XDJK2015B031, XDJK2018AA001),西南大学博士基金(No. SWU111081)资助
摘要:极管蛋白(Polar tube protein)是极管的主要成分,能特异性定位于微孢子虫极管,在微孢子虫侵染宿主过程中发挥重要作用。文中分析了家蚕微孢子虫极管蛋白1中潜在的O-、N-糖基化修饰位点,克隆了家蚕微孢子虫极管蛋白1全基因序列,并将其插入带有V5和His标签的真核表达载体pMT/Bip/V5-His A中,成功构建了pMT/Bip/V5-His A-NbPTP1重组质粒,经转染果蝇S2细胞后,发现NbPTP1基因能在果蝇细胞中高效表达。此外,Lectin blotting和β-消除反应分析结果表明:果蝇S2细胞内表达的NbPTP1具有O-糖基化修饰特征。以上结果为研究NbPTP1的糖基化修饰特征与其功能之间的关系提供了基础,有助于揭示微孢子虫侵染机制,建立可行有效的微孢子虫病诊断和防治措施。
关键词:极管蛋白1 果蝇S2细胞 糖基化 家蚕微孢子虫
Expression of Polar Tube Protein 1 (NbPTP1) from Nosema bombycis in Drosophila S2 cell lines and its glycosylation
Mengxian Long1, Yaoyao Tan1, Keke Liu1, Yujiao Wu1, Qing Lü1, Guoqing Pan1, Zeyang Zhou1,2


1 State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400715, China;
2 2 College of Life Sciences, Chongqing Normal University, Chongqing 400047, China
Received: December 4, 2017; Accepted: April 25, 2018
Supported by: National Natural Science Foundation of China (Nos. 31402138, 31601678), Chongqing Research Program of Basic Research and Frontier Technology (No. cstc2018jcyjAX0550), Fundamental Research Funds for the Central Universities (Nos. XDJK2015B031, XDJK2018AA001), Start-up Grant from Southwest University (No. SWU111081)
Corresponding author:Zeyang Zhou. Tel: +86-23-68251088; E-mail: zyzhou@swu.edu.cn
Abstract: The polar tube protein is the major component of polar tube, and can specifically locate on the polar tube of microsporidia and plays an important role in invasion host cell. In this study, we analyzed the potential O- and N- glycosylation sites in polar tube protein 1 from Nosema bombycis. NbPTP1 was successfully cloned to eukaryotic expression vector pMT/Bip/V5-His A, involved V5 and His tags. After transfection, NbPTP1 gene could be efficiently expressed in Drosophila S2 cells. In addition, Lectin blotting and beta elimination analysis showed that NbPTP1 expressed in Drosophila S2 cells was O-glycosylation. These studies provided a basis for understanding the relationship between glycosylation and function of NbPTP1, helped us to reveal the infection mechanism of microsporidia and established effective diagnosis and prevention methods for microsporidia.
Keywords: polar tube protein 1 Drosophila S2 cells glycosylation Nosema bombycis
微孢子虫是一类宿主广泛的细胞内专性寄生的单细胞真核生物[1-3]。迄今已发现约有200个属,1 400多种微孢子虫[4-5],然而不同种属微孢子虫拥有相似的侵染器官和侵染机制。未侵染宿主的微孢子虫,其极管能有序地盘绕在孢子内部;侵染宿主时,成熟孢子能感知外界环境变化并触发极管,长而中空的极管具有强力弹射和类似“注射器”的功能,能在不足两秒时间内完成整个弹出、刺入宿主细胞、运输孢原质的过程[6-8]。
极管蛋白(Polar tube protein)能特异定位于微孢子虫极管上,是组装极管的重要成分。目前发现至少存在5种主要的极管蛋白(PTP1、PTP2、PTP3、PTP4和PTP5),其中PTP1的含量约占极管蛋白总量的70%[5, 9-12]。不同来源的PTP1溶解性惊人相似,它们易溶于DTT、β-巯基乙醇等还原剂,不溶于SDS、尿素、氯仿等有机溶剂[12-15];然而其氨基酸序列同源性较低,脯氨酸和半胱氨酸残基的位置和数量却相对保守。脯氨酸是一个疏水性的氨基酸,其环状结构可在多肽中形成紧密结构使肽链具有一定的硬度,PTP1蛋白序列中高丰度的脯氨酸能使蛋白质具有如胶原蛋白、弹性蛋白一样的抗张强度、弹力和收缩力,这一蛋白特性将有助于微孢子虫的极管弹出和孢原质输出[4, 16]。
微孢子虫在侵染宿主过程中,超微结构观察发现极管刺入宿主细胞时,宿主细胞膜会发生内陷,但此过程中极管与宿主细胞膜之间互作的分子机制尚未阐明[9, 17]。微孢子虫能瞬间完成整个侵染过程,其中宿主细胞膜上一定存在能识别极管的受体,最新的研究发现海伦微孢子虫极管蛋白4 (EhPTP4)能与宿主表面的转铁蛋白受体发生相互作用从而介导微孢子虫对宿主的侵染[18]。Xu等发现海伦微孢子虫EhPTP1能结合凝集素ConA,且经PNGase F酶处理后的EhPTP1仍能与ConA结合,说明PTP1具有O-糖基化修饰而不具有N-糖基化修饰,并且兔脑炎微孢子虫EcPTP1能够被3H-Mannose标记,说明EcPTP1是一个O-甘露糖基化修饰蛋白[19-22]。利用甘露糖处理兔肾细胞RK13后,海伦微孢子虫对其侵染率明显低于未处理组,说明甘露糖封闭了宿主细胞表面的甘露糖结合位点,使微孢子虫极管上的PTP1蛋白无法结合宿主表面受体而降低了微孢子虫的感染率[19]。
家蚕微孢子虫是微孢子虫属的典型种,其快速的水平传播和垂直传播所引发的家蚕微粒子病给我国乃至世界养蚕业造成巨大经济损失。前期我们已经成功地获得了家蚕微孢子虫NbPTP1原核表达蛋白及其抗体[23],为了更好地研究具有翻译后修饰的家蚕微孢子虫极管蛋白1的功能,本研究采用果蝇S2表达系统,真核表达具有糖基化修饰的NbPTP1。对极管蛋白1开展深入研究有利于揭示微孢子虫侵染的关键机制,建立行之有效的微孢子虫病诊断和防治措施。
1 材料与方法1.1 材料pMT/Bip/V5-His A载体、果蝇S2细胞购自Invitrogen公司;菌种E. coli DH5α为本实验室保存;Taq酶、T4 DNA连接酶购自TaKaRa公司;质粒提取试剂盒、胶回收试剂盒购自Qiagen公司;各种限制性内切酶购自NEB公司;HRP-ConA购于Sigma公司;NbPTP1兔源多克隆抗体由本实验室制备,V5鼠源单克隆抗体购自Abcam公司;X-tremeGENE HP DNA转染试剂购自Roche公司;DNA Marker、ECL蛋白Marker购自北京全式金生物技术有限公司;引物合成与DNA测序由生工生物工程(上海)股份有限公司进行。家蚕微孢子虫Nosema bombycis CQ1分离株,由家蚕基因组生物学国家重点实验室病原微生物学研究组分离,保存于中国兽医微生物菌种保藏管理中心(CVCC),保藏号:CVCC102059。
1.2 糖基化修饰位点分析利用在线工具NetOGlyc 4.0 Server (http://www.cbs.dtu.dk/services/NetOGlyc/)和NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/)在家蚕微孢子虫数据库中SilkPathDB (http://silkpathdb.swu.edu.cn/)分别分析家蚕微孢子虫极管蛋白1序列中O-糖基化修饰位点和N-糖基化修饰位点。
1.3 表达载体的构建根据获得的家蚕微孢子虫极管蛋白基因序列,利用Primer 5.0软件设计特异引物F (5′-GG AAGATCTAACATGGCATGTTCACCCGG-3′,下划线为BglⅡ酶切位点)和引物R (5′-CCGCTCGAGGTTTAGCACACATGGATTAT-3′,下划线为XhoⅠ酶切位点),以家蚕微孢子虫基因组DNA为模板进行PCR扩增。反应条件为:94 ℃ 4 min;94 ℃ 45 s,58 ℃ 45 s,72 ℃ 1 min,30个循环;72 ℃ 10 min;4 ℃终止。PCR产物经1%琼脂糖凝胶电泳分离后,用胶回收试剂盒回收目的条带。将回收的PCR产物与载体pMD19-T连接,转化E. coli DH5α进行T克隆。对阳性克隆pMD19- NbPTP1进行双酶切和PCR验证后送生工生物工程(上海)股份有限公司测序。
分别用Bgl Ⅱ和XhoⅠ双酶切pMD19-NbPTP1质粒和pMT/Bip/V5-His A质粒,琼脂糖凝胶电泳分离后,胶回收试剂盒分别回收NbPTP1基因片段和pMT/Bip/V5-His A载体片段,将基因与载体片段连接后,转化E. coli DH5α进行亚克隆。对阳性克隆进行pMT/Bip/V5-His A-NbPTP1双酶切和PCR验证。
1.4 重组质粒转染细胞与融合蛋白诱导表达利用无内毒素的质粒提取试剂盒抽提重组表达质粒pMT/Bip/V5-His A-NbPTP1和空载对照pMT/Bip/V5-His A。按照产品说明书进行质粒转染,过程如下:分别将2 μg DNA质粒(pMT/Bip/ V5-His A-NbPTP1和空载对照pMT/Bip/V5-His A)溶于200 μL无血清的Schneider’s DrosophilaMedium中,混匀后,将制备好的DNA与6 μL脂质体轻轻混合均匀,即为包裹好的脂质体DNA,室温放置30 min;制备好的脂质体/DNA小心滴加至S2细胞表面(2.5×106个细胞/孔),置于28 ℃培养箱孵育24 h。转染24 h后,加入终浓度500 μmol/L的CuSO4进行蛋白的诱导表达,转染72 h后收集转染细胞。
1.5 融合蛋白鉴定别收集转染重组质粒pMT/Bip/V5-His A-NbPTP1和空载质粒pMT/Bip/V5-His A的细胞,经3 000 r/min离心5 min,分离细胞上清和沉淀。沉淀溶于RIPA缓冲液中,4 ℃溶解10 min后,12 000 r/min离心10 min取上清。采用12% SDS-PAGE和Western blotting检测NbPTP1基因在果蝇S2细胞中的表达。
1.6 融合蛋白的糖基化修饰分析Lectin blotting分析将细胞表达融合蛋白样品经12% SDS-PAGE后, 通过电转移方法将蛋白转移到PVDF膜上,膜用含2% (V/V) Tween-20的PBS缓冲液室温封闭2 min;将膜用PBS缓冲液洗2次;用1 μg/mL的HRP-Con A室温孵育16 h;用PBS缓冲液洗去多余的HRP-Con A,ECL显色。
参考Xu等[19]的β-消除方法,利用5 μL的0.1 mol/L NaOH溶液,分别与适量的家蚕微孢子虫天然PTP1蛋白和果蝇S2细胞表达的PTP1融合蛋白混合,在45 ℃下反应20 min和30 min后,置冰上并迅速加入1 μL 10%醋酸溶液终止反应。Western blotting和Lectin blotting方法分析NbPTP1在家蚕微孢子虫和果蝇S2细胞中糖基化修饰特征。利用Image J软件分析蛋白质含量,设反应0 min的蛋白含量为100%。
2 结果与分析2.1 NbPTP1糖基化修饰位点分析根据糖基化修饰位点分析结果(图 1)发现,NbPTP1蛋白序列上存在3个潜在的O-糖基化位点,分别为位于22位氨基酸的甲硫氨酸、位于26位和108位氨基酸的脯氨酸;其序列中无任何N-糖基化位点。依据前期报道[23],NbPTP1蛋白序列的N端前20个氨基酸组成了信号肽,去除基因信号肽后的融合蛋白表达质粒pMT/Bip/ V5-His A-NbPTP1并不影响其蛋白的翻译后修饰,应存在3个潜在的O-糖基化修饰位点,而无潜在的N-糖基化修饰位点。
 |
| 图 1 NbPTP1氨基酸序列分析 Figure 1 Amino acid sequence of NbPTP1. The signal peptide was marked in grey background, and the potential O-glycosylation sites were highlighted in bold. |
| 图选项 |
2.2 融合蛋白表达载体的构建根据家蚕微孢子虫基因组数据库,由于NbPTP1基因内不存在内含子,因此以家蚕微孢子虫基因组DNA为模板,利用特异性引物进行PCR扩增,获得长度约为1 200 bp的NbPTP1基因。并将获得的DNA片段与载体pMD19-T连接后,转化大肠杆菌DH5α,菌落PCR扩增验证正确后,通过基因测序进一步确认克隆构建成功。将构建成功的重组质粒pMD19-NbPTP1与载体pMT/Bip/V5-His A通过BglⅡ和XhoⅠ双酶切后连接,转化大肠杆菌DH5α,利用PCR和双酶切的方法对阳性重组质粒进行验证(图 2),结果发现阳性重组质粒pMT/Bip/V5-His A-NbPTP1中目的基因片段、载体片段与理论大小一致。
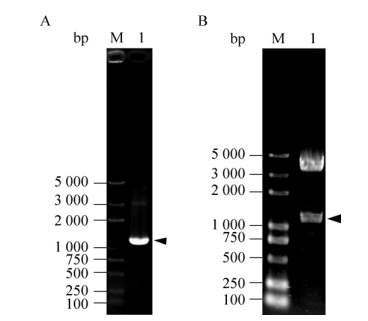 |
| 图 2 重组质粒pMT/Bip/V5-His A-NbPTP1的检测 Figure 2 Analysis of pMT/Bip/V5-His A-NbPTP1 recombinant plasmid. (A) PCR identification of pMT/Bip/V5-His A-NbPTP1. M: DNA marker; 1: PCR product. (B) Digestion identification of pMT/Bip/V5-His A-NbPTP1 with BglⅡand XhoⅠ. |
| 图选项 |
2.3 融合蛋白诱导表达及可溶性分析将融合蛋白表达重组质粒pMT/Bip/V5-His A-NbPTP1和空载对照质粒pMT/Bip/V5-His A瞬时转染果蝇S2细胞,经CuSO4诱导后, Western blotting检测结果见图 3。由图 3A可知,NbPTP1抗体能特异性识别重组质粒转染果蝇S2细胞后的胞内成分,分子量大小约为55 kDa,比理论分子量偏大约15 kDa,暗示其蛋白进行了翻译后修饰。而无论是空载对照质粒转染的细胞上清表达和胞内表达,还是重组质粒转染的细胞上清表达均无识别信号,说明pMT/Bip/V5-His A-NbPTP1瞬时转染S2细胞后只在细胞内表达,未分泌到细胞上清中。此外,经一抗二抗去除液处理后,PVDF膜上的NbPTP1抗体已被成功洗脱。随后利用V5抗体对同一PVDF膜上的蛋白质进行杂交,发现V5抗体也能识别果蝇S2细胞中胞内表达的融合蛋白,且与NbPTP1抗体杂交的条带一致(图 3B),说明果蝇S2细胞内表达的NbPTP1融合蛋白带有V5标签,由于V5标签和His标签位于同一开放阅读框内,暗示His标签也成功融合表达在重组蛋白中,可用于后续融合蛋白纯化。
 |
| 图 3 Western blotting分析NbPTP1在果蝇S2细胞中的表达 Figure 3 Western blotting analysis of NbPTP1 expressed in Drosophila S2 cell lines. (A) Analysis NbPTP1 expression by NbPTP1 polyclonal antibody. M: protein molecular weight marker; 1: supernatant from pMT/Bip/V5-His A transfected S2 cells; 2: pellet from pMT/Bip/V5-His A transfected S2 cells; 3: supernatant from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells; 4: pellet from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells. (B) Analysis NbPTP1 expression by V5 monoclonal antibody. M: protein molecular weight marker; 1: supernatant from pMT/Bip/V5-His A transfected S2 cells; 2: pellet from pMT/Bip/V5-His A transfected S2 cells; 3: supernatant from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells; 4: pellet from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells. |
| 图选项 |
2.4 融合蛋白的糖基化修饰分析根据NbPTP1序列分析发现其具有潜在的糖基化修饰特征,利用辣根过氧化物酶(HRP)标记的ConA,结合Lectin blotting检测方法,分析果蝇S2细胞内表达的NbPTP1融合蛋白糖基化修饰特征。由图 4A可知,HRP标记的ConA可以在转染重组质粒和空载质粒的细胞上清及胞内表达物中分别识别多条具有糖基化修饰的蛋白条带。但被ConA识别的蛋白条带中,仅有55 kDa蛋白条带最清晰,且蛋白浓度最大。经一抗、二抗去除液处理后,PVDF膜上的HRP-ConA已被成功洗脱。随后利用V5抗体对同一PVDF膜上的蛋白质进行杂交,结合图 4B的Western blotting检测结果,此55 kDa蛋白条带既能被HRP标记的ConA识别也能被V5标签抗体识别,说明该55 kDa蛋白条带为融合表达的NbPTP1蛋白,而其他未能被V5抗体识别的条带应为果蝇S2细胞自身表达的糖蛋白。因此,融合表达的NbPTP1蛋白具有糖基化修饰。
 |
| 图 4 Lectin blotting分析果蝇S2细胞中表达NbPTP1的糖基化修饰 Figure 4 Lectin blotting analysis the glycosylation of NbPTP1 expressed in Drosophila S2 cell lines. (A) Analysis the glycosylation of NbPTP1 by HRP-ConA antibody. M: protein molecular weight marker; 1: supernatant from pMT/Bip/V5-His A transfected S2 cells; 2: pellet from pMT/Bip/V5-His A transfected S2 cells; 3: supernatant from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells; 4: pellet from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells. (B) Analysis NbPTP1 expression by V5 monoclonal antibody. 1: supernatant from pMT/Bip/V5-His A transfected S2 cells; 2: pellet from pMT/Bip/V5-His A transfected S2 cells; 3: supernatant from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells; 4: pellet from pMT/Bip/V5-His A-NbPTP1 transfected S2 cells. |
| 图选项 |
2.5 融合蛋白的O-糖基化特征分析前期研究发现海伦微孢子虫EhPTP1和脑炎微孢子虫EcPTP1均具有O-糖基化修饰,而不具有N-糖基化修饰[19-22];同时我们发现家蚕微孢子虫天然极管蛋白1具有O-糖基化修饰特征(未发表)。因此,利用β-消除法对果蝇S2细胞内表达的NbPTP1融合蛋白O-糖基化修饰进行了分析。实验结果发现,不论是天然的NbPTP1还是果蝇S2细胞表达的NbPTP1融合蛋白,经过0.1 mol/L NaOH处理不同时间后,PTP1总蛋白量有所减少;经一抗、二抗去除液处理后,Lectin blotting检测结果发现PTP1蛋白中的糖蛋白含量也会随反应时间延长而降低,但糖消除速度明显快于蛋白降解速度(图 5)。说明果蝇S2细胞表达的NbPTP1与天然PTP1一样,均具有O-糖基化修饰,且ConA可以结合在O-糖链上。
 |
| 图 5 家蚕微孢子虫天然NbPTP1与果蝇S2细胞中表达NbPTP1的O-糖基化修饰特征 Figure 5 Analysis of O-glycosylation of NbPTP1. (A) NbPTP1 from Nosema bombycis was treated with NaOH. (B) NbPTP1 expressed in Drosophila S2 cell lines was treated with NaOH After treatment, samples were analyzed by SDS-PAGE followed by either immunoblotting with polyclonal rabbit antibody (bottom panel) or labeled ConA overlay (top panel). |
| 图选项 |
3 讨论自1857年Nageli从病蚕中分离到第一个微孢子虫起,研究人员对微孢子虫的形态结构、分类、侵染机制等方面进行了大量研究。微孢子虫宿主域广、种类繁多,不同种属微孢子虫均拥有一个独特的侵染器官——极管。
极管蛋白1作为微孢子虫中第一个被鉴定的能定位于极管上的蛋白,其含量最为丰富,也是目前研究的最为透彻的极管蛋白。随着各微孢子虫基因组测序工作相继完成[24-25],发现各种属微孢子虫极管蛋白1的序列中N端均具有信号肽、富含脯氨酸、具有糖基化修饰等特征。研究发现,EhPTP1和EcPTP1均具有O-甘露糖基化修饰,并且证实极管蛋白1的糖基化修饰与微孢子虫对宿主的侵染有密切联系[9, 16-17]。蛋白质的糖基化修饰在许多病原真菌侵染宿主的过程中起着粘附、侵染和维持稳定等重要作用[26]。自然界中许多病原都具有类似侵染机制,如添加外源糖可以降低动物体内病原细菌E. coli的粘附宿主细胞能力,从而阻止病原侵染[27]。对于肠道原虫,它们大多利用自身表面糖蛋白粘附宿主上的粘膜细胞进而侵染宿主[28-29]。推测微孢子虫极管蛋白1的O-糖基化修饰可能在保护自身极管免受宿主细胞消化或与宿主细胞膜上的甘露糖受体发生相互作用的过程中发挥功能,从而促进微孢子虫对宿主的侵染[19, 30-31]。
由于蛋白溶解性极差,使得从微孢子虫中分离纯化天然极管蛋白1并进行后续功能研究变得十分艰难。另外,微孢子虫具有一层厚而坚固的孢壁,加之其严格的细胞内寄生,现有的遗传操作体系在微孢子虫体内均无法实施,因此,微孢子虫中蛋白质功能研究进展相对缓慢。为了更好地研究NbPTP1功能,前期我们已经成功地对其进行了原核表达,制备了相应的多克隆抗体,但原核表达的NbPTP1蛋白并不能真实反映家蚕微孢子虫极管蛋白的天然折叠及翻译后修饰特征,对后续研究其功能造成了阻碍。目前,家蚕微孢子虫也可以通过酵母表达系统异源表达相关蛋白,研究其功能[32];然而,酵母表达时常出现表达的蛋白发生自剪切的情况。与酵母表达系统相比,果蝇S2表达系统结合了哺乳动物细胞和昆虫表达系统的优点,培养条件要求低,生长周期短,是一种成本低廉、容易操作的高效表达系统。本研究利用果蝇S2细胞表达家蚕微孢子虫的极管蛋白1,能表达既具有天然构象、又具有翻译后糖基化修饰的蛋白,为后续NbPTP1的功能研究奠定基础。
本研究是首次利用果蝇S2异源真核表达系统表达家蚕微孢子虫的极管蛋白。结果表明,NbPTP1蛋白在果蝇S2细胞内成功表达,其分子量比理论分子量偏大约15 kDa,通常情况下发生翻译后修饰的蛋白质都会比其理论分子量大;并且表达的蛋白能够被ConA抗体识别,说明其具有糖基化修饰特征;β-消除反应后果蝇S2细胞表达的NbPTP1上糖链被消去的速度明显快于总蛋白的降解速度,说明果蝇S2细胞表达的NbPTP1融合蛋白与家蚕微孢子虫天然PTP1蛋白一致,都是O-糖基化蛋白;融合蛋白具有V5、His标签便于后续蛋白纯化。然而,此融合蛋白的表达还存在一些不足之处,需要在后续实验中改进:1)果蝇S2表达系统所用载体pMT/Bip/V5-His A中Bip序列是一个分泌型信号,能使表达的目的蛋白分泌到细胞上清中,无需裂解细胞就能获得大量的目的蛋白[33]。在昆虫表达系统中提高异源蛋白分泌表达的一大策略就是使用一个昆虫特异的信号肽来替代异源蛋白信号肽[34],然而并非所有信号肽的替换都能成功地将异源蛋白表达至细胞上清中[35],此外更换信号肽对某些特定蛋白质的分泌表达程度并无任何影响[36]。我们的实验结果发现,去除了目的蛋白NbPTP1的信号肽,仅靠Bip序列并不能将表达的目的蛋白分泌到细胞上清中,因此后续会优化信号肽序列与表达条件。2)可建立稳定表达NbPTP1的细胞系,让蛋白在细胞上清中持续分泌表达,利于大规模的连续生产[37-38]。3)可利用融合蛋白带有His标签的特征,对表达的NbPTP1蛋白进行分离纯化,进行后续糖基化修饰位点、糖链特征鉴定及其功能分析。
参考文献
| [1] | Sak B, Kotková M, Hlásková L, et al. Limited effect of adaptive immune response to control encephalitozoonosis.Parasite Immunol, 2017, 39(12): 12496.DOI: 10.1111/pim.2017.39.issue-12 |
| [2] | Liu H, Jiang ZH, Yuan ZY, et al. Infection by and genotype characteristics of Enterocytozoon bieneusi in HIV/AIDS patients from Guangxi Zhuang autonomous region, China.BMC Infect Dis, 2017, 17(1): 684.DOI: 10.1186/s12879-017-2787-9 |
| [3] | Long MX, Wu YJ, Chen J, et al. Research progress in polar tube proteins from microsporidia.Sci Sericult, 2014, 40(5): 917–923.(in Chinese). 龙梦娴, 吴玉娇, 陈洁, 等. 微孢子虫极管蛋白的研究进展.蚕业科学, 2014, 40(5): 917-923. |
| [4] | Han B, Weiss LM. Microsporidia: obligate intracellular pathogens within the fungal kingdom.Microbiol Spectr, 2017, 5(2): FUNK-0018-2016. |
| [5] | Weiss LM, Becnel JJ. Microsporidia: Pathogens of Opportunity.Oxford: Wiley-Blackwell, 2014. |
| [6] | Ohshima K. On the function of the polar filament of Nosema bombycis.Parasitology, 1937, 29(2): 220–224.DOI: 10.1017/S0031182000024768 |
| [7] | Weidner E. Ultrastructural study of microsporidian invasion into cells.Z Parasitenkd, 1972, 40(3): 227–242. |
| [8] | Frixione E, Ruiz L, Santillán M, et al. Dynamics of polar filament discharge and sporoplasm expulsion by microsporidian spores.Cell Motil Cytoskel, 1992, 22(1): 38–50.DOI: 10.1002/cm.v22:1 |
| [9] | Delbac F, Peuvel I, Metenier G, et al. Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species.Infect Immun, 2001, 69(2): 10160–1024. |
| [10] | Peuvel I, Peyret P, Méténier G, et al. The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi.Mol Biochem Parasitol, 2002, 122(1): 69–80.DOI: 10.1016/S0166-6851(02)00073-7 |
| [11] | Delbac F, Peyret P, Metenier G, et al. On proteins of the microsporidian invasive apparatus: complete sequence of a polar tube protein of Encephalitozoon cuniculi.Mol Microbiol, 1998, 29(3): 825–834.DOI: 10.1046/j.1365-2958.1998.00975.x |
| [12] | Keohane EM, Orr GA, Zhang HS, et al. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans.Mol Biochem Parasitol, 1998, 94(2): 227–236.DOI: 10.1016/S0166-6851(98)00071-1 |
| [13] | Weidner E. The microsporidian spore invasion tube. The ultrastructure, isolation, and characterization of the protein comprising the tube.J Cell Biol, 1976, 71(1): 23–34.DOI: 10.1083/jcb.71.1.23 |
| [14] | Keohane EM, Orr GA, Takvorian PM, et al. Purification and characterization of a microsporidian polar tube protein.Mol Biochem Parasitol, 1996, 79(2): 255–259.DOI: 10.1016/0166-6851(96)02666-7 |
| [15] | Keohane EM, Weiss LM. Characterization and function of the microsporidian polar tube: a review.Folia Parasitol (Praha), 1998, 45(2): 117–127. |
| [16] | Xu YJ, Weiss LM. The microsporidian polar tube: a highly specialised invasion organelle.Int J Parasitol, 2005, 35(9): 941–953.DOI: 10.1016/j.ijpara.2005.04.003 |
| [17] | Lom J. On the structure of the extruded microsporidian polar filament.Z Parasitenkd, 1972, 38(3): 200–213.DOI: 10.1007/BF00329598 |
| [18] | Han B, Polonais V, Sugi T, et al. The role of microsporidian polar tube protein 4 (PTP4) in host cell infection.PLoS Pathog, 2017, 13(4): e1006341.DOI: 10.1371/journal.ppat.1006341 |
| [19] | Xu YJ, Takvorian P M, Cali A, et al. Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans.Infect Immun, 2004, 72(11): 6341–6350.DOI: 10.1128/IAI.72.11.6341-6350.2004 |
| [20] | Bouzahzah B, Weiss LM. Glycosylation of the major polar tube protein of Encephalitozoon cuniculi.Parasitol Res, 2010, 107(3): 761–764.DOI: 10.1007/s00436-010-1950-7 |
| [21] | Xu YJ, Takvorian P, Cali A, et al. Lectin binding of the major polar tube protein (PTP1) and its role in invasion.J Eukaryot Microbiol, 2003, 50(6): 600–601. |
| [22] | Taupin V, Garenaux E, Mazet M, et al. Major O-glycans in the spores of two microsporidian parasites are represented by unbranched manno-oligosaccharides containing α-1, 2 linkages.Glycobiology, 2007, 17(1): 56–67. |
| [23] | Wu YJ, Long MX, Chen J, et al. Cloning and prokaryotic expression of Nosema bombycis polar tube protein 1 (NbPTP1).Sci Sericult, 2014, 40(2): 258–264.(in Chinese). 吴玉娇, 龙梦娴, 陈洁, 等. 家蚕微孢子虫极管蛋白1(NbPTP1)的基因克隆及原核表达.蚕业科学, 2014, 40(2): 258-264. |
| [24] | Pan GQ, Xu JS, Li T, et al. Comparative genomics of parasitic silkworm microsporidia reveal an association between genome expansion and host adaptation.BMC Genomics, 2013, 14: 186.DOI: 10.1186/1471-2164-14-186 |
| [25] | Katinka MD, Duprat S, Cornillot E, et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi.Nature, 2000, 414(6862): 450–453. |
| [26] | Varki A. Biological roles of oligosaccharides: all of the theories are correct.Glycobiology, 1993, 3(2): 97–130.DOI: 10.1093/glycob/3.2.97 |
| [27] | Aronson M, Medalia O, Schori L, et al. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl α-D-mannopyranoside.J Infect Dis, 1979, 139(3): 329–332.DOI: 10.1093/infdis/139.3.329 |
| [28] | Chen XM, LaRusso NF. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells.Gastroenterology, 2000, 118(2): 368–379.DOI: 10.1016/S0016-5085(00)70219-8 |
| [29] | Zhang Z, Duchêne M, Stanley SL Jr. A monoclonal antibody to the amebic lipophosphoglycan- proteophosphoglycan antigens can prevent disease in human intestinal xenografts infected with Entamoeba histolytica.Infect Immun, 2002, 70(10): 5873–5876.DOI: 10.1128/IAI.70.10.5873-5876.2002 |
| [30] | Peek R, Delbac F, Speijer D, et al. Carbohydrate moieties of microsporidian polar tube proteins are targeted by immunoglobulin G in immunocompetent individuals.Infect Immun, 2005, 73(12): 7906–7913.DOI: 10.1128/IAI.73.12.7906-7913.2005 |
| [31] | Polonais V, Prensier G, Méténier G, et al. Microsporidian polar tube proteins: highly divergent but closely linked genes encode PTP1 and PTP2 in members of the evolutionarily distant Antonospora and Encephalitozoon groups.Fungal Genet Biol, 2005, 42(9): 791–803.DOI: 10.1016/j.fgb.2005.05.005 |
| [32] | Lin LP, Pan GQ, Li T, et al. The protein import pore Tom40 in the microsporidian Nosema bombycis.J Eukaryot Microbiol, 2012, 59(3): 251–257.DOI: 10.1111/j.1550-7408.2012.00618.x |
| [33] | Yamashita A, Nango E, Ashikawa Y. A large-scale expression strategy for multimeric extracellular protein complexes using Drosophila S2 cells and its application to the recombinant expression of heterodimeric ligand-binding domains of taste receptor.Protein Sci, 2017, 26(11): 2291–2301.DOI: 10.1002/pro.3271 |
| [34] | Ailor E, Pathmanathan J, Jongbloed JDH, et al. A bacterial signal peptidase enhances processing of a recombinant single chain antibody fragment in insect cells.Biochem Biophys Res Commun, 1999, 255: 444–450.DOI: 10.1006/bbrc.1999.0233 |
| [35] | Tessier DC, Thomas DY, Khouri HE, et al. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide.Gene, 1991, 98(2): 177–183.DOI: 10.1016/0378-1119(91)90171-7 |
| [36] | Jarvis DL, Summers MD, Garcia A Jr, et al. Influence of different signal peptides and prosequences on expression and secretion of human tissue plasminogen activator in the baculovirus system.J Biol Chem, 1993, 268(22): 16754–16762. |
| [37] | Shen X, Dojcinovic D, Baldi L, et al. Improved process conditions for increasing expression of MHC class Ⅱ protein from a stable Drosophila S2 cell line.Biotechnol Lett, 2018, 40(1): 85–92.DOI: 10.1007/s10529-017-2440-0 |
| [38] | Zitzmann J, Weidner T, Czermak P. Optimized expression of the antimicrobial protein Gloverin from Galleria mellonella using stably transformed Drosophila melanogaster S2 cells.Cytotechnology, 2017, 69(2): 371–389.DOI: 10.1007/s10616-017-0068-5 |
