

1 河南科技大学 动物科技学院 动物疫病与公共卫生重点实验室,河南 洛阳 471003
2 洛阳职业技术学院,河南 洛阳 471003
Construction and characterization of type III secretion system of attenuated Salmonella typhimurium
Chuan Yu1, Chongkai Zhai1, Chengshui Liao1, Yu Zuhua1, Lei He1, Yanyan Jia1, Jing Li1, Chunjie Zhang1, Xiangchao Cheng1,2


1 The Key Laborary of Animal Disease and Public Health, College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471003, Henan, China
2 Luoyang Vocational & Technical College, Luoyang 471003, Henan, China
Received: April 7, 2016; Accepted: June 28, 2016
Supported by:National Natural Science Foundation of China (Nos. 31302059, 31572489), Scientific and Technological Project of Henan Province (No. 152102110078), Doctor Foundation of Henan University of Science and Technology (No. 4025-13480064)
Corresponding authors:Xiangchao Cheng. Tel/Fax: +86-379-64179396; E-mail: chengxch@126.com
Abstract: In order to develop a recombinant attenuated Salmonella typhimurium as oral live vaccine vector, we constructed recombinant plasmid pYA-sopENt100 by replacing thetrcpromoter with thesopEpromoter and secretion signal sequence sopENt100of Salmonella typhimuriumon the basis of plasmid pYA3493. Then, the complementary plasmid pYA-sopENt100 was transformed into ΔcrpΔasdSL1344 by electroporation to generate attenuated Salmonella typhimurium type III secretion system ΔcrpΔasdSL1344 (pYA-sopENt100). We further characterized ΔcrpΔasdSL1344 (pYA-sopENt100). We also constructed a recombinant strain ΔcrpΔasdSL1344 (pYA-sopENt100-egfp) that harbored the reporter gene-enhanced green fluorescent protein (egfp) gene. Vero cells were infected with ΔcrpΔasdSL1344 (pYA-sopENt100-egfp) and the ability of delivery foreign antigens was tested via Western blotting analysis. The results of PCR, enzyme digestion and sequencing showed that the ΔcrpΔasdSL1344 (pYA-sopENt100) type III secretion system was constructed successfully. The serotype of ΔcrpΔasdSL1344 (pYA-sopENt100) was identical to ΔcrpΔasdSL1344 and SL1344. Compared with wild strain SL1344, the biochemical characteristics of ΔcrpΔasdSL1344 (pYA-sopENt100) had obvious change, but it was basically the same with ΔcrpΔasdSL1344. The growth speed was much slower than that of the wild strain SL1344. The chicken virulence test (LD50) showed that the virulence of ΔcrpΔasdSL1344 (pYA-sopENt100) was 7×104 times lower than SL1344. In addition, we observed the 37 kDa SopENt100-egfp protein in the cultured supernatant of ΔcrpΔasdSL1344 (pYA-sopENt100-egfp) strain by Western blotting analysis. However, both the 37 kDa SopENt100-egfp protein and 27 kDa EGFP protein were detected in ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)-infected Vero cells. These results demonstrated that the recombinant Salmonella typhimuriumtype III secretion system ΔcrpΔasdSL1344 (pYA-sopENt100) was successfully constructed, and it should be used as a live vaccine vector for expressing foreign genes.
Key words: attenuated Salmonella typhimurium type III secretion system sopE gene balance-lethal system live vaccine vector
鼠伤寒沙门菌属肠杆菌科沙门菌属的B血清群成员,具有广泛感染的宿主谱,也是一种重要的人畜共患病原菌,在医学、兽医学和公共卫生上均具有十分重要的意义[1-2]。通过基因工程减毒的鼠伤寒沙门菌毒力降低,但能保持良好的免疫原性,能有效地刺激机体产生粘膜、细胞和体液免疫应答[3-4]。另外,减毒沙门菌LPS作为内在佐剂,可以刺激宿主细胞释放各种细胞因子以及增强树突状细胞捕获和加工MHC-I类抗原的能力,有利于将重组沙门菌的抗原呈递给特异性的B、T细胞[5-6]。因此,减毒鼠伤寒沙门菌是携带抗原的良好载体和天然粘膜免疫佐剂,这已受到医学与兽医学的广泛关注[7-8]。
沙门菌质粒平衡致死载体系统的建立有效解决了外源质粒的不稳定携带和表达以及耐药基因潜在的生物安全性问题[9-10]。asd质粒平衡致死系统是基于缺失载体菌的编码二氨基庚二酸(DL-α,ε-Diaminopimelicacid,DAP)合成酶的基因而开发的载体表达系统。asd基因(Aspartic semialdehyde dehydrogenase)编码的天冬氨酸β-半乳糖脱氢酶是DAP生物合成途径中的必需酶,asd突变株在无外源DAP条件下不能形成完好的细胞壁,最终溶菌死亡。然而外源的抗原基因可被克隆至asd+的质粒中与突变菌形成互补,使重组菌在无外源DAP存在的条件下仍能存活[11-12]。本实验室为开发新型鼠伤寒沙门菌口服活疫苗载体,在缺失编码cAMP受体蛋白(cAMP receptor protein,crp)基因[13]的沙门菌基础上成功构建出减毒鼠伤寒沙门菌平衡致死系统ΔcrpΔasdSL1344 (pYA-3493)[14]。
鼠伤寒沙门菌Ⅲ型分泌系统(type Ⅲ secretion system,T3SS)与细菌的定殖、侵染以及在宿主细胞内的存活有着密切的关系[15]。T3SS通过一个复杂的针头复合物将外源蛋白注入宿主细胞内,从而调节细胞的功能。沙门菌外膜蛋白E (Salmonella outer protein E,sopE)是T3SS的一种重要的功能蛋白,它通过与Cdc42和Rac1相互作用,激活Rho GTPase,从而导致膜波动和肌动蛋白细胞骨架重组,促进沙门菌内化到宿主细胞[16]。此外,已经证实sopE氨基末端100个氨基酸(sopENt100)是分泌和转位区域,能够作为一种外源蛋白运输工具,可以高效输送异源蛋白到真核细胞胞质[17]。另外,在真核细胞内SopE蛋白能够迅速地被泛素化处理和被蛋白酶体降解,不仅有利于外源抗原的递呈,而且对宿主细胞自身的毒性较小[18]。
综上所述,本试验在减毒鼠沙门菌平衡致死系统的基础上,利用鼠伤寒沙门菌III型分泌系统高效递呈外源抗原的优势,构建减毒鼠伤寒沙门菌ΔcrpΔasdSL1344 (pYA-sopENt100)三型分泌表达系统,进一步研究该系统递呈外源抗原的能力及其生物学特性,进而为研发更加安全、有效、稳定的新型重组减毒鼠伤寒沙门菌口服活疫苗载体奠定基础。
1 材料与方法1.1 菌株、质粒、细胞及培养条件鼠伤寒沙门菌SL1344强毒株购自中国兽医药品监督所,质粒pEGFP-N1购自Invitrogen公司,无抗性原核表达载体pYA3493 (asd+,pBRori,β-lactamase signal sequence)及其宿主菌χ6097 (araΔ(lac-pro) rPslΔasdA4Δ[zhf-2:: Tn10] thiф80d/lacZΔM15)由美国华盛顿大学Dr. Roy Curtiss Ⅲ教授惠赠,鼠伤寒沙门菌ΔcrpΔasdSL1344由本室构建保存[14],大肠杆菌和鼠伤寒沙门菌在各种培养基中37 ℃静置或振摇培养,各培养基根据需要加入终浓度为50 μg/mL DAP、50 μg/mL的庆大霉素。
1.2 主要试剂、培养基和实验动物沙门菌属诊断血清及各种单因子血清购自宁波天润生物药业有限公司;DAP购自Sigma-Aldrich公司;Taq DNA聚合酶、琼脂糖、dNTPs、DNA marker、T4 DNA连接酶和各种限制性内切酶购自大连宝生物工程有限公司;胰蛋白胨、酵母提取物购自英国Oxoid公司;鼠抗EGFP单抗及HPR标记兔抗鼠IgG购自Abcam;质粒小量提取试剂盒购自北京鼎国昌盛生物技术有限公司;凝胶回收试剂盒购自生工生物工程有限公司;麦康凯琼脂和生化鉴定试剂均购自杭州天和微生物试剂有限公司;胎牛血清和DMEM培养基购自Gibco;1日龄健康罗曼雏鸡,按常规方法检测均为沙门菌抗体阴性[19]。
1.3 引物设计与合成根据鼠伤寒沙门菌(GenBank Accession No. AE008859) sopE基因的启动子和分泌信号序列以及质粒pEGFP-N1中EGFP的全基因序列,运用Primer 5.0引物设计软件设计引物并由北京鼎国昌盛生物技术有限责任公司合成(表 1,引物中的斜体部分为酶切位点)。
表 1 PCR扩增所用的引物序列Table 1 The primer sets for PCR in this study
| Primer name | Primer sequence (5'-3') |
| Fw-PsopE | CCGGAATTAATTCTTCAATGCCAGAA CGGCAAGGCTC |
| Rw-PsopE | CCGGAATTCCGCACTACCTCTAATATC |
| Fw-egfp | CGGAATTCATGGTGAGCAAGGGCG |
| Rw-egfp | CCCAAGCTTTTACTTGTACAGCTC |
表选项
1.4 沙门菌三型分泌表达载体的构建及鉴定以质粒pYA3493为基础,用鼠伤寒沙门菌sopE基因的启动子及分泌信号序列替代原有的Ptrc启动子,构建沙门菌三型分泌表达载体pYA-sopENt100 (图 1);将egfp报告基因插入pYA-sopENt100下游,构建重组标记载体pYA-sopENt100-egfp。然后分别将质粒pYA-sopENt100和pYA-sopENt100-egfp转化大肠杆菌χ6097,取100 μL涂布于LB平板上。15?18 h后挑取无色单菌落接种于LB液体培养基进行扩大培养,用碱裂解法提取质粒进行PCR、酶切和测序鉴定。
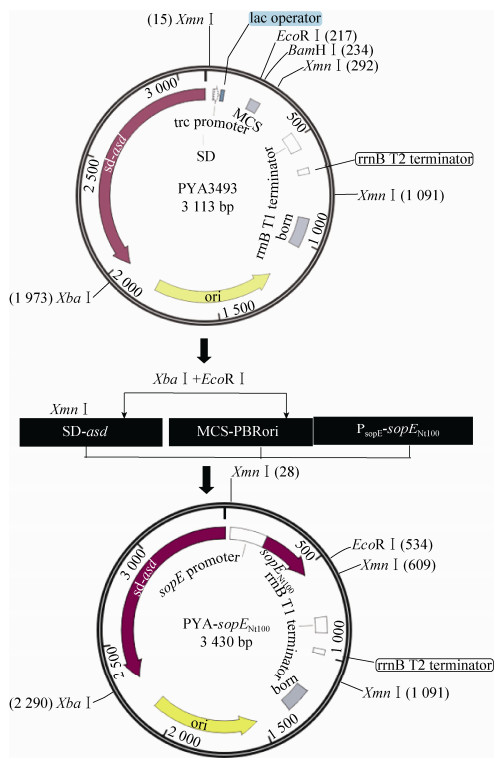 |
| 图 1 重组质粒pYA-sopENt100构建示意图 Figure 1 Construction of the recombinant plasmid pYA-sopENt100. |
| 图选项 |
1.5 重组菌株的构建及鉴定10 μL pYA-sopENt100和pYA-sopENt100-egfp质粒加入100 μL Δcrp?asdSL1344的电转化感受态中混合均匀,冰浴30 min,将混合物迅速转移至电极杯中,然后进行电击;电击后迅速加入400 μL预热的LB液体培养基,37 ℃、180×g振荡培养3 h,取出100 μL涂布于不含DAP的LB平板上。15?24 h后挑取无色单菌落接种于LB液体培养基中,用碱裂解法提取质粒进行PCR和酶切鉴定。
1.6 重组菌株ΔcrpΔasdSL1344(pYA-sopENt100-egfp)生物学特性1.6.1 重组菌株的表型及生化鉴定重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)及其亲本株?crpSL1344、双缺失株Δcrp?asdSL1344和野生株SL1344分别划线接种于含1%麦芽糖的麦康凯琼脂培养基和LB琼脂培养基,培养15 h后进行O和H抗原等血清型鉴定,然后将各菌株分别转接于各生化管中培养研究其生化特性。
1.6.2 重组质粒在菌株中的稳定性检测重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)在LB固体板上划线培养,挑取单菌落于LB液体培养基中培养,按1:100的比例转接到LB±DAP液体培养基中培养,连续培养50代。培养产物每10代挑取50个单菌落提取质粒,并进行PCR扩增鉴定和计算质粒丢失率[20],以研究重组质粒在菌株中的遗传稳定性。
1.6.3 重组菌株的生长特性鉴定分别挑取重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)及其亲本株?crpSL1344、双缺失株Δcrp?asdSL1344和野生株SL1344单菌落培养过夜,菌液用无菌PBS连续10倍稀释,选取合适稀释度涂板计数,计算母液细菌浓度。然后菌液以终浓度均为1×106 CFU/mL进行转接,连续培养18 h,每隔1 h测定波长600 nm的光密度(OD600)值,绘制4种菌株的生长曲线。
1.6.4 重组菌株对雏鸡的毒力试验无菌挑取重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)及其亲本株ΔcrpSL1344和野生株SL1344单菌落,培养至对数生长期时选择合适稀释度,每个稀释度口服10只鸡,每只200 μL,另设PBS对照组,观察30 d,记录鸡死亡情况,运用生物软件(Bliss)计算各组LD50。
1.7 SopENt100-egfp融合基因在重组菌株中的表达情况鉴定挑取减毒鼠伤寒沙门菌ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)的单菌落于LB液体培养基中,37 ℃培养12 h;然后按1:100的体积比转接LB培养基继续培养8 h,8 000×g离心10 min收集沉淀和上清。沉淀用超声波进行破碎,上清液经0.22 μm滤膜过滤,加等体积预冷的20%三氯乙酸冰浴20 min,8 000×g离心30 min;然后将浓缩后上清用1 mL无水乙醇洗涤,12 000×g离心5 min;重复洗涤2次,向上清和沉淀中加入5×SDS上样缓冲液进行SDS-PAGE分析,并进一步进行Western blotting分析。第一抗体为鼠抗EGFP单抗(1:1 000稀释),第二抗体为HPR标记兔抗鼠IgG (1:3 000稀释),同时设对照组Δcrp?asdSL1344 (pYA-sopENt100)。
1.8 重组减毒沙门菌侵染Vero细胞及表达蛋白的Western blotting分析培养Vero细胞生长至80%?90%时,按细菌/细胞感染比500:1分别将重组减毒鼠伤寒沙门菌Δcrp?asdSL1344 (pYA-sopENt100-egfp)和Δcrp?asdSL1344 (pYA-sopENt100)加入六孔板中,培养4 h后,用无菌PBS洗涤3次,加入含有10%胎牛血清及50 μg/mL庆大霉素的DMEM培养基,继续培养24 h后,收集细胞,用细胞裂解液裂解细胞,4 ℃、12 000×g离心5 min收集上清液,加入等体积的2×SDS上样缓冲液,混匀后煮沸5 min进行SDS-PAGE,并进一步进行Western blotting分析,其中一抗为鼠抗EGFP单抗(1:1 000稀释),二抗为HPR标记的兔抗鼠IgG (1:3 000稀释)。
2 结果与分析2.1 沙门菌三型分泌表达载体的构建及鉴定对pYA-sopENt100进行PCR和酶切鉴定,PCR扩增出大小约为500 bp的目的条带(图 2A);经Acc Ⅲ和EcoRⅠ双酶切后出现大小分别为500 bp和2 900 bp的两条目的条带(图 2B);pYA-sopENt100-egfp可扩增出约800 bp的egfp片段(图 2C),经EcoRⅠ和Hind Ⅲ双酶切后出现大小分别约800 bp和3 400 bp两条带(图 2D)。测序结果表明,PsopE-sopENt100和egfp基因序列与预期标准核酸序列的同源性均为100%。重组质粒可在大肠杆菌χ6097 (asd-)菌株中稳定存在,以上结果表明,鼠伤寒沙门菌三型分泌表达载体pYA-sopENt100及其重组载体pYA-sopENt100-egfp构建成功。
 |
| 图 2 重组质粒的鉴定 Figure 2 Identification of the recombinant plasmid. (A) PCR identification of recombinant plasmid pYA-sopENt100. M: DNA marker ladder (DL 5 000); 1: recombinant plasmid pYA-sopENt100; 2: ddH2O control. (B) Restriction endonuclease digestion of recombinant plasmid pYA-sopENt100. M: DNA marker ladder (DL 5 000); 1: pYA-sopENt100. (C) PCR identification of recombinant plasmid pYA-sopENt100-egfp. M: DNA marker ladder (DL 2 000); 1: pYA-sopENt100-egfp; 2: ddH2O control. (D) Restriction endonuclease digestion of recombinant plasmid pYA-sopENt100-egfp. M: DNA marker ladder (DL 5 000); 1: recombinant plasmid pYA-sopENt100-egfp. |
| 图选项 |
2.2 重组菌株的构建及鉴定重组菌株Δcrp?asdSL1344 (pYA-sopENt100)和Δcrp?asdSL1344 (pYA-sopENt100-egfp)能够在不含DAP的LB固体培养基上生长,初步说明重组质粒pYA-sopENt100和pYA-sopENt100-egfp导入了宿主菌Δcrp?asdSL1344。重组质粒pYA-sopENt100经PCR可以扩增出500 bp的目的条带,经Acc Ⅲ和EcoR Ⅰ双酶切后出现大小分别为500 bp和2 900 bp的两条带,而重组质粒pYA-sopENt100-egfpPCR可以扩增出800 bp的目的条带,经EcoR Ⅰ和Hind Ⅲ双酶切后出现大小分别约为800 bp和3 400 bp的两条带。以上结果可说明重组质粒pYA-sopENt100和pYA-sopENt100-egfp被成功导入了宿主菌株Δcrp?asdSL1344。
2.3 ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)重组菌株的生物学特性2.3.1 重组菌株的表型及生化鉴定血清型鉴定表明,重组菌Δcrp?asdSL1344 (pYA-sopENt100-egfp)的血清型同亲本株和野生株相比没有发生变化,血清型仍为O1, 4, 5, 12:Hi1, 2。野生株SL1344能够利用麦芽糖,在含有麦芽糖的麦康凯琼脂培养基上呈红色菌落;而重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)不能利用麦芽糖,因此在含麦芽糖的麦康凯琼脂培养基上呈无色菌落,这与亲本株ΔcrpSL1344保持一致。生化鉴定结果显示,重组菌株的生化特性与野生株SL1344相比发生了明显改变,其失去了利用丙三醇、阿拉伯糖、麦芽糖、山梨醇和木糖碳源的能力,也不能分解H2S,但仍保留了利用葡萄糖、甘露醇的能力,这些都与亲本株ΔcrpSL1344基本保持一致。
2.3.2 重组质粒在菌株中的稳定性检测将重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)在LB±DAP液体培养基中连续培养50代,培养产物每隔10代挑取50个单菌落进行PCR扩增鉴定并计算质粒丢失率。质粒稳定性结果表明,重组菌株连续培养50代后,LB组菌株均可扩增出约800 bp的egfp目的条带(图 3),质粒保存率为100%,而LB+DAP组菌株的质粒丢失率为48% (图 4)。
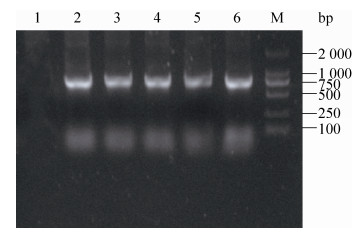 |
| 图 3 重组沙门菌体外稳定性PCR鉴定结果 Figure 3 PCR identification of the stabilities of recombinant plasmid in recombinant strains. M: DNA marker ladder (DL 2 000); 1: ddH2O control; 2?6: the 10th, 20th, 30th, 40th and 50th generation of ΔcrpΔasdSL1344 (pYA-sopENt100-egfp). |
| 图选项 |
 |
| 图 4 重组质粒在沙门菌中的稳定性鉴定结果 Figure 4 The plasmid stabilities in recombinant strains. |
| 图选项 |
2.3.3 重组菌株的生长特性鉴定重组菌株Δcrp?asdSL1344 (pYA-sopENt100-egfp)、缺失菌Δcrp?asdSL1344和亲本株ΔcrpSL1344的菌落大小明显小于野生株SL1344,其平均菌落直径大小分别为0.39 mm、0.36 mm、0.42 mm和0.79 mm。以取样时间为横坐标,以每小时所测菌液OD600值为纵坐标,绘制4种细菌的生长曲线(图 5)。结果表明,重组菌株的生长速度低于强毒株,而与缺失菌株和亲本株相比没有明显变化。
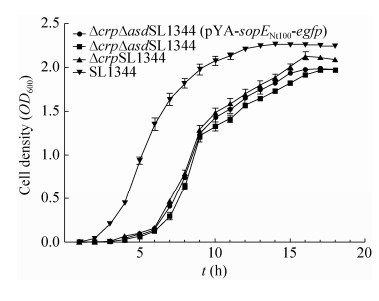 |
| 图 5 ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)、ΔcrpΔasdSL1344、ΔcrpSL1344和SL1344生长曲线 Figure 5 Growth curves of ΔcrpΔasdSL1344 (pYA-sopENt100-egfp), ΔcrpΔasdSL1344, ΔcrpSL1344 and SL1344. |
| 图选项 |
2.3.4 重组菌株对雏鸡的毒力试验各试验组1日龄雏鸡接种后连续观察30 d,计算各组死亡情况和半数致死量(LD50)。由表 2可知,口服感染?crp?asdSL1344 (pYA-sopENt100-egfp)的LD50是2.28×1010 CFU,ΔcrpSL1344的LD50是1.14×1010 CFU,野生菌株SL1344的LD50是3.25×105 CFU,重组菌株Δcrp?asdSL1344(pYA-sopENt100-egfp)的LD50较野生株SL1344降低了7.0×104倍,与?crpSL1344保持相当。试验结果表明重组菌?crp?asdSL1344 (pYA-sopENt100-egfp)毒力明显减弱,作为疫苗载体安全性较好。
表 2 各实验组口服感染结果Table 2 Oral infection results of each experimental group
| Strains | Inoculating dose (CFU) | Death No./ Total chickens | LD50 (CFU) |
| ΔcrpΔasdSL1344 (pYA-sopENt100) | 4.8×1010 | 6/10 | 2.28×1010 |
| 4.8×109 | 3/10 | ||
| 4.8×108 | 0/10 | ||
| 4.8×107 | 0/10 | ||
| ΔcrpSL1344 | 5.4×1010 | 8/10 | 1.14×1010 |
| 5.4×109 | 3/10 | ||
| 5.4×108 | 0/10 | ||
| 5.4×107 | 0/10 | ||
| SL1344 | 5.1×108 | 10/10 | 3.25×105 |
| 5.1×107 | 10/10 | ||
| 5.1×106 | 9/10 | ||
| 5.1×105 | 6/10 | ||
| PBS | 500 μL | 0/10 | / |
表选项
2.4 重组菌株的表达产物鉴定重组减毒鼠伤寒沙门菌ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)经过培养后,Western blotting分析显示,在约37 kDa处有SopENt100-egfp融合蛋白条带,并且存在于过滤的上清和沉淀中(图 6),表明SopENt100-egfp融合蛋白能够在减毒鼠伤寒沙门菌ΔcrpΔasdSL1344中获得分泌表达。
 |
| 图 6 重组菌株ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)表达产物的Western blotting鉴定 Figure 6 Western blotting identification of the expressed protein from recombinant attenuated strain ΔcrpΔasdSL1344 (pYA-sopENt100-egfp). M: protein marker; 1: supernatant of ΔcrpΔasdSL1344 (pYA-sopENt100); 2: supernatant of ΔcrpΔasdSL1344 (pYA-sopENt100-egfp); 3: precipitation of ΔcrpΔasdSL1344 (pYA-sopENt100); 4: precipitation of ΔcrpΔasdSL1344(pYA-sopENt100-egfp). |
| 图选项 |
2.5 重组减毒沙门菌侵染Vero细胞及Western blotting分析重组减毒沙门菌ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)感染Vero细胞24 h后,收集细胞经处理后进行Western blotting反应。结果显示,在约37 kDa处有SopENt100-egfp融合蛋白条带,约27 kDa处出现EGFP蛋白条带,而空载体菌株ΔcrpΔasdSL1344 (pYA-sopENt100)在对应位置并未出现条带,表明该重组三型分泌表达系统能够有效地将SopENt100-egfp融合蛋白递呈Vero细胞,并且SopENt100-egfp融合蛋白能够被Vero细胞加工处理,其分泌相关蛋白SopENt100能够被进一步切除(图 7)。
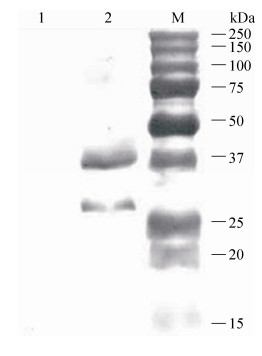 |
| 图 7 重组菌株感染Vero细胞后EGFP蛋白的Western blotting鉴定 Figure 7 Western blotting identification of the expressed EGFP protein from Vero cells infected by recombinant attenuated strain. M: protein marker; 1: ΔcrpΔasdSL1344 (pYA-sopENt100); 2: ΔcrpΔasdSL1344 (pYA-sopENt100-egfp). |
| 图选项 |
3 讨论减毒鼠伤寒沙门菌可通过自然感染途径如口服或鼻内接种,引起动物较强的黏膜、细胞和体液免疫应答,具有培养条件简单、繁殖速度较快、免疫原性良好、制备及使用方便、经济等多种优点,可以作为携带外源基因的良好载体,具有广泛应用前景[12, 17]。减毒沙门菌的生物安全性和稳定性直接决定着疫苗的安全性和适用性。我们前期的研究发现,口服1.0×109CFU的减毒沙门菌ΔcrpΔasdSL1344 (pYA3493)对鸡只没有明显的毒性[14]。这表明减毒沙门菌ΔcrpΔasdSL1344具有作为疫苗活载体的潜能。
利用减毒沙门菌作为活载体表达外源抗原,研制多价疫苗时,常常出现减毒沙门菌在体内恶劣环境压力下质粒丢失或表达不稳定的现象。目前,已报道多种方法来提高外源基因的稳定表达,其中,效果最稳定且应用最多的是asd+质粒平衡表达系统。asd+质粒平衡表达系统由缺失其生存所必需的asd基因的亲本菌株和含有asd基因的互补质粒组成。asd基因表达产物天冬氨酸β-半乳糖脱氢酶,是DAP合成的必需酶,而DAP又是革兰氏阴性菌细胞壁的主要成分。因此,asd基因的缺失将导致突变菌株无法在DAP-条件下生存。沙门菌质粒平衡致死载体系统的建立有效解决了外源质粒的不稳定携带和表达以及耐药基因潜在生物安全性问题[11-12]。为保证该系统的稳定性,重组质粒pYA-sopENt100上的SD-asd基因为asd基因的部分片段,其缺少相应的启动子元件(-10区和-35区),仅保留了SD序列。同时,重组质粒pYA-sopENt100采用pBR ori低水平复制起始位点,这样一方面大大降低了Asd蛋白的表达水平,减少了Asd蛋白对宿主菌的毒性作用。另一方面能够适量增加质粒的拷贝数,从而提高外源蛋白的表达量[21]。质粒稳定性结果显示,重组菌株连续培养50代,LB组菌株均可扩增出约800 bp的egfp目的条带,质粒保存率为100%,而LB+DAP组菌株的质粒丢失率为48%。这初步表明该分泌表达系统在DAP-环境下具有良好的稳定性。
减毒沙门菌通过利用细菌的分泌系统将外源抗原递呈给抗原递呈细胞。在疫苗载体设计的过程中,外源抗原的表达和递呈能力直接关系着疫苗的功效。鼠伤寒沙门菌进入宿主细胞后形成内化空泡而不能有效地将外源抗原呈递给MHC分子,从而直接影响着鼠伤寒沙门菌的抗原递呈能力[22]。T3SS通过一个复杂的针头复合物将外源蛋白注入宿主细胞内,从而发挥相应的生物学功能[23]。许多****已经证实,利用鼠伤寒沙门菌三型分泌系统来递呈外源抗原能够有效的解决上述问题,具有更大的优越性和广泛的应用价值。将外源抗原与鼠伤寒沙门菌三型分泌蛋白SopE的分泌和转位信号进行融合,从而利用沙门菌三型分泌系统来实现外源蛋白的定向运输[17]。另外,在真核细胞内SopE蛋白能够迅速地被蛋白酶体降解,有利于外源抗原的递呈[18]。2011年Chen等[24]通过构建sopE-Sj23LHD-GST表达载体来预防日本血吸虫,试验结果表明该载体能够有效地递呈sopE-Sj23LHD-GST,对日本血吸虫具有良好的免疫保护力;2012年Juárez等[25]构建sopE-ESAT-6等表达载体来预防结核分枝杆菌,结果表明该载体可以诱使机体产生较高的特异性抗体,可以有效地预防结核病。鉴于此,本研究将标记基因egfp插入sopE信号序列下游,初步探究构建的三型分泌表达载体pYA-sopENt100抗原递呈能力。结果显示,重组减毒沙门菌ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)成功表达了融合蛋白SopENt100-egfp。此外,重组菌株ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)感染Vero细胞后,Western blotting结果发现,在约37 kDa处有SopENt100-egfp融合蛋白条带,而在约27 kDa处出现EGFP蛋白条带。上述结果表明,本研究构建的减毒鼠伤寒沙门菌三型分泌系统ΔcrpΔasdSL1344 (pYA-sopENt100)能够有效地递呈外源抗原,并且融合蛋白中的信号蛋白SopENt100能够被宿主细胞进一步切除。
本研究通过对重组减毒鼠伤寒沙门菌的生物学特性研究发现,该重组菌株与强毒株相比生长速度明显减慢,毒力明显降低,具有良好的安全性,且遗传稳定。这说明sopE基因的表达对重组菌株的生物学特性没有明显的影响。同时,重组减毒鼠伤寒沙门菌ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)表达的SopENt100-egfp融合蛋白可存在于过滤的上清中,说明重组菌株可分泌性表达该蛋白,避免了被胞内蛋白酶降解,利于表达蛋白的正确折叠及保持良好的生物学活性。以上结果表明将鼠伤寒沙门菌三型分泌系统引入沙门菌平衡致死系统可以作为安全、稳定、高效表达外源基因的口服重组活疫苗载体,具有广阔的应用前景。
综上,本研究利用减毒鼠伤寒沙门菌三型分泌系统以及平衡致死系统成功构建了能稳定携带外源抗原的新型减毒鼠伤寒沙门菌三型分泌表达系统ΔcrpΔasdSL1344 (pYA-sopENt100);重组菌ΔcrpΔasdSL1344 (pYA-sopENt100-egfp)毒力降低,遗传稳定,能分泌性表达SopENt100-egfp融合蛋白,且能有效地向Vero细胞递呈EGFP蛋白,为开发减毒鼠伤寒沙门菌新型口服活疫苗载体奠定基础。
参考文献
| [1] | Jawale CV, Lee JH. Characterization of a Salmonella typhimurium ghost carrying an adjuvant protein as a vaccine candidate for the protection of chickens against virulent challenge.Avian Pathol, 2014, 43(6): 506–513.DOI: 10.1080/03079457.2014.966303 |
| [2] | Bagheryan Z, Raoof JB, Golabi M, et al. Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample.Biosens Bioelectron, 2016, 80: 566–573.DOI: 10.1016/j.bios.2016.02.024 |
| [3] | Tang LH, Pan ZM, Cheng NN, et al. Construction and immunogenicity of attenuated Salmonella typhimurium harbouring stable DNA vaccine against H5 subtype of avian influenza virus.Acta Microbiol Sin, 2007, 47(4): 662–666.(in Chinese). 唐丽华, 潘志明, 程宁宁, 等. 稳定携带H5亚型禽流感病毒候选DNA疫苗减毒沙门氏菌的构建及其免疫原性.微生物学报, 2007, 47(4): 662-666. |
| [4] | Cao J, Chen ZH, Ren YL, et al. Oral immunization with attenuated Salmonella carrying a co-expression plasmid encoding the core and E2 proteins of hepatitis C virus capable of inducing cellular immune responses and neutralizing antibodies in mice.Vaccine, 2011, 29(20): 3714–3723.DOI: 10.1016/j.vaccine.2011.02.083 |
| [5] | Nandre RM, Lee JH. Construction of a recombinant-attenuated Salmonella enteritidis strain secreting Escherichia coli heat-labile enterotoxin B subunit protein and its immunogenicity and protection efficacy against salmonellosis in chickens.Vaccine, 2014, 32(3): 425–431.DOI: 10.1016/j.vaccine.2013.10.054 |
| [6] | Chen DS, Guo WZ, Xu ZW, et al. Construction of a dual-promoter expression plasmid delivered by Salmonella choleraesuis C500.Chin J Biotech, 2009, 25(3): 341–347.(in Chinese). 陈弟诗, 郭万柱, 徐志文, 等. 猪霍乱沙门氏菌递送的双启动子表达载体的构建.生物工程学报, 2009, 25(3): 341-347. |
| [7] | Lin IYC, Van TTH, Smooker PM. Live-attenuated bacterial vectors: tools for vaccine and therapeutic agent delivery.Vaccines, 2015, 3(4): 940–972.DOI: 10.3390/vaccines3040940 |
| [8] | Zhang D, Huang XB, Zhang XH, et al. Construction of an oral vaccine for transmissible gastroenteritis virus based on the TGEV N gene expressed in an attenuated Salmonella typhimurium vector.J Virol Methods, 2016, 227: 6–13.DOI: 10.1016/j.jviromet.2015.08.011 |
| [9] | Zhang Q, Ma QL, Li Q, et al. Enhanced protection against nasopharyngeal carriage of Streptococcus pneumoniae elicited by oral multiantigen DNA vaccines delivered in attenuated Salmonella typhimurium.Mol Biol Rep, 2011, 38(2): 1209–1217.DOI: 10.1007/s11033-010-0219-7 |
| [10] | Kim SW, Kang HY, Hur J, et al. Construction of a conditional lethal Salmonella mutant via genetic recombination using the ara system and asd gene.J Microbiol Methods, 2011, 87(2): 202–207.DOI: 10.1016/j.mimet.2011.08.004 |
| [11] | Li J, Chen SB, Yu ZH, et al. Construction of host-vector balanced lethal system of Salmonella typhimurium SL1344Δcya mutant and immune protection test of chickling.Acta Microbiol Sin, 2015, 55(7): 942–948.(in Chinese). 李静, 陈松彪, 余祖华, 等. 鼠伤寒沙门菌SL1344株环化腺苷酸合成酶缺失株平衡致死系统的构建及其雏鸡免疫保护试验.微生物学报, 2015, 55(7): 942-948. |
| [12] | Xu YD, Guo AZ, Liu WH, et al. Construction and characterization of ΔcrpΔasd mutant host-vector balanced lethal system of Salmonella choleraesuis C500 Strain.Chin J Biotech, 2006, 22(3): 366–372.(in Chinese). 徐引弟, 郭爱珍, 刘维红, 等. 猪霍乱沙门氏菌C500株ΔcrpΔasd缺失株平衡致死载体系统的构建及鉴定.生物工程学报, 2006, 22(3): 366-372. |
| [13] | Liao CS, Cheng XC, Zhao ZQ, et al. Construction and characterization of the cAMP receptor protein gene deletion mutant of Salmonella typhimurium SL1344 strain.Chin J Vet Sci, 2011, 31(12): 1711–1716.(in Chinese). 廖成水, 程相朝, 赵战勤, 等. 鼠伤寒沙门菌SL1344株cAMP受体蛋白基因缺失株的构建及其生物学特性.中国兽医学报, 2011, 31(12): 1711-1716. |
| [14] | Tian FH, Cheng XC, Zhang CJ, et al. Construction and characterization of a Salmonella typhimurium SL1344ΔcrpΔasd mutant strain.Chin J Vet Sci, 2013, 33(11): 1700–1706.(in Chinese). 田芳华, 程相朝, 张春杰, 等. 鼠伤寒沙门菌SL1344株ΔcrpΔasd缺失株的构建及生物学特性初步研究.中国兽医学报, 2013, 33(11): 1700-1706. |
| [15] | Galán JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells.Science, 1999, 284(5418): 1322–1328.DOI: 10.1126/science.284.5418.1322 |
| [16] | Friebel A, IIchmann H, Aepfelbacher M, et al. SopE and SopE2from Salmonella typhimurium activate different sets of Rho GTPases of the host cell.J Biol Chem, 2001, 276(36): 34035–34040.DOI: 10.1074/jbc.M100609200 |
| [17] | Chen LM, Briones G, Donis RO, et al. Optimization of the delivery of heterologous proteins by the Salmonella enterica serovar typhimurium type III secretion system for vaccine development.Infect Immun, 2006, 74(10): 5826–5833.DOI: 10.1128/IAI.00375-06 |
| [18] | Kubori T, Galán JE. Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation.Cell, 2003, 115(3): 333–342.DOI: 10.1016/S0092-8674(03)00849-3 |
| [19] | Ministry of Health of the People's Republic of China. GB/T 14926.1-2001 Laboratory animal-method for examination of Salmonella sp.Beijing: China Standards Press, 2002(in Chinese). 中华人民共和国卫生部. GB/T 14926.1-2001实验动物沙门菌检测方法.北京: 中国标准出版社, 2002. |
| [20] | Nakayama K, Kelly SM, Curtiss R III. Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain.Nat Biotechnol, 1988, 6(6): 693–697.DOI: 10.1038/nbt0688-693 |
| [21] | Wang SF, Kong QK, Curtiss R III. New technologies in developing recombinant attenuated Salmonella vaccine vectors.Microb Pathog, 2013, 58(5): 17–28. |
| [22] | Panthel K, Meinel KM, Sevil Domènech VE, et al. Salmonella type III-mediated heterologous antigen delivery: a versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors.Int J Med Microbiol, 2008, 298(1/2): 99–103. |
| [23] | Cangelosi C, Hannagan S, Santiago CP, et al. Transfer of the cloned Salmonella SPI-1 type III secretion system and characterization of its expression mechanisms in gram negative bacteria in comparison with cloned SPI-2.Microbiol Res, 2015, 180: 57–64.DOI: 10.1016/j.micres.2015.07.006 |
| [24] | Chen G, Dai Y, Chen JX, et al. Oral delivery of the Sj23LHD-GST antigen by Salmonella typhimurium type III secretion system protects against Schistosoma japonicum infection in mice.PLoS Negl Trop Dis, 2011, 5(9): e1313.DOI: 10.1371/journal.pntd.0001313 |
| [25] | Juárez-Rodríguez MD, Yang J, Kader R, et al. Live attenuated Salmonella vaccines displaying regulated delayed lysis and delayed antigen synthesis to confer protection against Mycobacterium tuberculosis.Infect Immun, 2012, 80(2): 815–831.DOI: 10.1128/IAI.05526-11 |
