崔鸿亮, 刘长莉

 , 李春雅, 宋志峰, 杨雅静, 王炎伟
, 李春雅, 宋志峰, 杨雅静, 王炎伟 东北林业大学生命科学学院, 黑龙江 哈尔滨 150040
收稿日期:2020-11-07;修回日期:2021-02-15;网络出版日期:2021-06-07
基金项目:教育部扶贫专项(2572020DF02)
*通信作者:刘长莉, E-mail: liuchangli@nefu.edu.cn.
摘要:单一微生物降解水稻秸秆效果不明显,多种微生物组合在一起的微生物菌群能够有效降解水稻秸秆,是当前降解秸秆类废物的首要选择。[目的] 探究微生物菌群比单一菌株转化秸秆效率提高的种间协作机制,为改善秸秆类物质的生物降解过程提供理论参考。[方法] 通过菌种组合,以水稻秸秆减重率为指标人工构建出降解效果优于单菌培养的微生物群体组合,利用分子生物学方法对实验菌株进行鉴定,结合纤维素酶活性、发酵产物GC-MS分析等指标得出水稻秸秆降解时微生物群体的种间协作机制。[结果] 菌株B(Bacillus cereus)在30℃培养8 d水稻秸秆降解率为50.9%,菌株B与菌株D2(Bacillus amyloliquefaciens)、W1(Ochrobactrum intermedium)、G1降解(Bacillus licheniformis)组合构成4株菌BD2W1G1的复合菌群,在30℃培养8 d水稻秸秆降解率为73.3%,比B单菌株分解能力提高22.4%。发酵产物分析结果表明,菌株B单独培养产生大量酸、酚等物质。B+D2联合培养,发酵液内酸类相对减少87.4%,酚类相对减少61.9%(十五烷酸、正十六烷酸、2,4二叔丁基酚和6-叔丁基对甲酚减少明显),水稻秸秆降解率为64.6%。当B+D2+W1联合培养,发酵液内酚类进一步减少15.7%(6-叔丁基对甲酚减少明显),水稻秸秆降解率为71.0%。当B+D2+W1+G1联合培养,酚类继续减少10.7%,水稻秸秆降解率为73.3%。[结论] 十五烷酸、正十六烷酸、2,4二叔丁基酚和6-叔丁基对甲酚等酸、酚类物质会抑制菌株B分解水稻秸秆的效率,菌株D2、W1、G1能够减少包括4种物质在内的酸、酚类含量(酸类共减少82.9%,酚类共减少88.2%),来提高分解效率(共提升22.4%的分解效果)。水稻秸秆成分复杂,具有不同功能的菌种组合成复合菌剂,减少反馈抑制形成代谢互补,能有效提高水稻秸秆生物降解效果。
关键词:水稻秸秆微生物降解菌群菌种功能
Analysis on the mechanism of synergistic improvement of rice straw transformation by microbial flora
Hongliang Cui, Changli Liu

 , Chunya Li, Zhifeng Song, Yajing Yang, Yanwei Wang
, Chunya Li, Zhifeng Song, Yajing Yang, Yanwei Wang College of Life Sciences, Northeast Forestry University, Harbin 150040, Heilongjiang Province, China
Received: 7 November 2020; Revised: 15 February 2021; Published online: 7 June 2021
*Corresponding author: Changli Liu, E-mail: liuchangli@nefu.edu.cn.
Foundation item: Supported by the Key Poverty Alleviation Project of the Ministry of Education (2572020DF02)
Abstract: The effect of a single microorganism in degrading rice straw is not apparent. The microbial flora, combined with multiple microorganisms, can effectively degrade rice straw, which is currently the primary choice for straw waste degradation. [Objective] To explore the interspecies coordination mechanism, the microbial flora can transform rice straw more efficiently than a single strain and provide a theoretical reference for improving the biodegradation process of straw materials. [Methods] A microbial population combination with a degradation effect better than monoculture was constructed artificially through the combination of strains and the weight loss of rice straw as an indicator. The experimental strains were identified by molecular biology methods. Combined with cellulase activity and GC-MS analysis of fermentation products revealed the interspecies coordination mechanism of microbial populations during rice straw degradation. [Results] Strain B (Bacillus cereus) cultured at 30℃ for 8 days, the degradation rate of rice straw was 50.9%. The combination of strain B and strain D2 (Bacillus amyloliquefaciens), W1 (Ochrobactrum intermedium), and G1 (Bacillus licheniformis) constituted 4 strains of BD2W1G1. For the composite flora, rice straw's degradation rate by culturing at 30℃ for 8 days is 73.3%, 22.4% higher than that of single strain B. The fermentation product analysis showed that strain B produced a large amount of acid, phenol, and other substances in a single culture. B+D2 co-culture, the degradation rate of rice straw is 64.6%, the acid in the fermentation broth is relatively reduced by 87.4%, and the phenol is reduced by 61.9% (pentadecanoic acid, n-hexadecanoic acid, 2, 4 Di-tert-butylphenol, and 6-tert-butyl p-cresol is significantly reduced). When B+D2+W1 is co-cultured, the fermentation broth phenols are further reduced by 15.7% (6-tert-butyl-p-cresol is significantly reduced), and rice straw is degraded by 71.0%. When B+D2+W1+G1 is co-cultured, phenols and acids continue to decrease by 10.7%, and rice straw is degraded by 73.3%. [Conclusion] Pentadecanoic acid, n-hexadecanoic acid, 2, 4-di-tert-butylphenol and 6-tert-butyl-p-cresol, and other acids phenols can inhibit the efficiency of strain B in decomposing rice straw. Strain D2, W1, and G1 can reduce the content of acids and phenols, including the four substances (A total of 82.9% reduction in acids and 88.2% reduction in phenols), improve the decomposition efficiency (A total of 22.4% increase in decomposition effect). The composition of rice straw is complex, and the bacteria with different functions are combined into a compound inoculum, which reduces feedback inhibition and forms metabolic complementarity, which can effectively improve the biodegradation effect of rice straw.
Keywords: rice strawmicrobial degradationmicrobial consortiastrain function
单一微生物降解未经过任何预处理或灭菌的水稻秸秆,效果并不明显,由多种微生物组合在一起的微生物菌剂已经成为当前降解秸秆类废物的首要选择[1]。自然界中,绝大多数微生物物种通过相互作用而共存,甚至一些微生物只有在和某种生物体结合后才能发挥出最大效果,如产甲烷系统中氢在物种之间的转移[2],卤代化合物的异种生物降解[3]等。显然,微生物有时会缺乏一些关键代谢途径,而其他微生物的代谢途径可能会对其进行补充[4-6]。大量研究表明,功能不同的各种菌种组合在一起分工协作是降解水稻秸秆的理想选择[7-9],与单一培养方法(即单个菌株)降解相比,微生物群体的降解潜力更大[10-12]。Jiménez等[13]提出的“内源性异养演替”也指出,秸秆降解时,先锋种群出现启动秸秆解构,随着代谢产物增多,利用代谢产物的物种会逐渐繁殖并进行进一步代谢。因此,在处理潜在环境波动时,混合种群将执行单个物种难以甚至不可能完成的功能[14]。这些生态学原理是木质纤维素降解微生物菌群更高有效性的基础,目前正被用于设计能够有效执行一系列秸秆降解过程的微生物群体[15]。
目前,微生物群体在水稻秸秆分解过程中,菌株间如何进行协同,各菌种具体发挥什么作用,哪些代谢产物阻碍了水稻秸秆分解过程,仍然了解不够全面。为进一步了解微生物群体在分解水稻秸秆时菌种间的作用关系及各种代谢产物对水稻秸秆分解效果的影响,以能够分解水稻秸秆的好氧菌株B (Bacillus cereus)为基础,采用还原筛选法与生态策略相结合的方法,利用从环境样本中获得的大量菌株与B进行一一组合逐个筛选,人工构建出能够显著提高水稻秸秆降解效果的微生物群体并分析其作用机理,将系统内各个菌株和代谢产物的作用逐个分析,了解微生物菌群中菌种间相关代谢特性,改善秸秆类物质的糖化过程[16-17],为进一步提高水稻秸秆的生物降解效果提供理论参考。
1 材料和方法 1.1 菌种来源 自黑龙江省漠河市林区、哈尔滨市、五常镇采集牛粪、腐烂秸秆及秸秆下土样,取回后将不同样点的粪便和土壤混合,筛选纤维素分解菌。
1.2 培养基 筛选培养基(g/L):秸秆粉/羧甲基纤维素钠/微晶纤维素/D-水杨苷5,酵母粉2,KH2PO4 0.5,MgSO4·7H2O 0.5,琼脂20。
固氮培养基(g/L):KH2PO4 0.2,K2HPO4 0.8,MgSO4·7H2O 0.2,CaSO4·2H2O 0.1,FeCl3微量,Na2MoO4·2H2O微量,酵母粉0.5,甘露醇20,琼脂15,pH 7.2。
实验培养基(g/L):水稻秸秆8,酵母粉2,KH2PO4 0.5,MgSO4·7H2O 0.5。
1.3 接菌方式 (1) 菌培养时接种5 mL,(2) 菌培养时每株菌各接种2.5 mL,(3) 菌培养时每株菌各接种1.66 mL,(4)菌培养时每株菌各接种1.25 mL。
1.4 菌株组合方法 以分解效率最高的菌株B为基础,根据还原筛选与生态策略相结合的方法与纤维素分解菌和固氮菌一一组配筛选,选取水稻秸秆分解率上升效果最大的菌种组合进行下一轮组配,直至分解效果微量提升或不再提升后停止组配。
1.5 菌株鉴定 将菌株培养至对数生长期,利用细菌DNA提取试剂盒(北京庄盟试剂公司),按使用说明书提取细菌基因组DNA。以菌种总DNA为模板,利用细菌通用引物27F (5′-AGAGTTTGATCCTGGCTC AG-3′)和1492R (5′-GGTTACCTGTTACGT-3′)进行PCR扩增。扩增后产物送交生物公司测序以获得实验菌株的16S rRNA序列。所得序列结果在NCBI中进行BLAST分析,获得与该菌的16S rRNA序列同源性最高的已知序列,并从GenBank数据库中调取相似性较高的相关菌株基因序列构建系统发育树。
1.6 秸秆降解量 培养样品经6000 r/min离心10 min,除去上清液后用稀酸冲洗以消除菌体,继续离心10 min,70 ℃烘干后称重,初秸秆质量与剩余秸秆质量之差为秸秆降解质量,实验中每个样本设置3个重复,取平均值。
1.7 纤维素酶活测定 纤维素酶的活性测定参照国际理论与应用化学联合会(IUPAC)推荐的测定纤维素酶活性方法[18-19]:培养基内取发酵液3 mL,10000 r/min、离心10 min,取0.5 mL上清液,加入到含有1%的微晶纤维素/D-水杨苷/CMC-Na和1.5 mL柠檬酸缓冲液(0.05 mol/L,pH 4.5)的试管中,30 ℃水浴30 min,采用DNS法测定还原糖含量。实验中每个样本设置3个重复。以1 h催化纤维素水解生成1 μmol葡萄糖所需的酶量定义为一个酶活力单位U,以U/mL表示。
1.8 氨氮含量测定 利用连华科技公司的水质监测仪测定培养基内氨氮含量,实验中每个样本设置3个重复,取平均值。
1.9 分解产物GC-MS分析 样品处理:取0.1 g秸秆分解产物,加1 mL甲醇,充分振荡,静置12 h后,用注射器挤出汁液,过0.22 μm孔径的微孔过滤器,取1 μL进样测定。气质联用条件:测定仪器及测定条件:使用日本岛津产QP 5050型气质联机(GC-MS)测定。分析柱:CP-Chirasil-Dex CB型毛细管柱(25 m× 0.25 mm);载气为氦气,流速为1.0 mL/min,进样口温度250 ℃。柱温初始温度50 ℃,保持2 min,以6 ℃/min升至280 ℃、保持10 min。分流进样,分流比10:1。MS:电子能量70 ev (EI),电荷质量范围m/z:40–600。扫描速度1 scan/s。电离温度230 ℃。接口温度250 ℃[20-21]。
1.10 代谢产物绝对定量 按照表 1方法配置4种代谢产物的标准液,标准液进行GC-MS分析后建立峰面积与含量标准曲线,以确定4种代谢产物的绝对含量。
表 1. 4种代谢物标准曲线测定配比 Table 1. Four kinds of metabolites standard curve determination ratio
| Number | Standard solution/% | Pentadecanoic acid/g | n-Hexadecanoic acid/g | 2, 4 Di-tert-butylphenol/g | 6-tert-butyl-p-cresol/g | Methanol/mL |
| 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| 1 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 100 |
| 2 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 100 |
| 3 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 100 |
| 4 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 100 |
| 5 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 100 |
表选项
1.11 数据处理 利用GraphPad Prism 6软件作数据图,图表中数据为平均值±标准差,Excel软件制作标准曲线,MEGA 5.2软件制作系统发育树。
2 结果和分析 2.1 纤维素分解菌的分离和筛选 用4种不同碳源的筛选培养基分离筛选土样中纤维素分解菌,共得到19株细菌。用固氮培养基分离筛选土样中固氮菌,共得到3株细菌。筛选材料及菌种代号如表 2所示。
表 2. 筛选培养基碳源及菌株代号 Table 2. Screening materials and experimental strain codes
| Straw powder | Microcrystalline cellulose | D-Salicin | CMC-Na | Nitrogen fixation medium |
| F1 | W1 | D1 | C1 | G1 |
| F2 | W2 | D2 | C2 | – |
| F3 | W3 | D3 | C3 | G2 |
| F4 | W4 | D4 | C4 | – |
| – | W5 | D5 | C5 | G3 |
| –: None. | ||||
表选项
菌株筛选后对各纤维素分解菌进行水稻秸秆降解效果和纤维素酶活(AC酶活、CB酶活,CMC酶活)测定,对固氮菌进行氨氮含量测量,结果如图 1所示。实验发现,筛选出的19株纤维素分解菌除菌株W2外,都能不同程度上降解水稻秸秆,但水稻秸秆降解效率仍是菌株B最高,菌株D2的分解效果其次。纤维素酶活方面,D2菌株的AC酶活为18.97 U/mL,CB酶活为6.09 U/mL,为19株菌中最高。菌株F1的CMC酶活最高,为13.71 U/mL。对G1、G2和G3的氨氮含量测量发现,3株菌的氨氮含量均有上升,其中,G1菌株在培养5 d时培养基中氨氮含量为837.43 mg/L,随后趋于平稳,氨氮含量上升缓慢但后期平稳。G2菌株在培养2 d时培养基中氨氮含量达到最大,为898.13 mg/L,随后开始下降,氨氮含量上升迅速下降也迅速。G3菌株氨氮上升幅度小,3 d时达到405 mg/L后趋于平稳。说明筛选的19株纤维素分解菌具有分解纤维素能力,3株固氮菌也都具有固氮能力。
 |
| 图 1 十九株纤维素分解菌的各纤维素酶活及水稻秸秆降解率对比和3株固氮菌培养基中氨氮含量变化 Figure 1 Comparison of cellulase activity and degradation rate of rice straw of 19 cellulolytic bacteria and changes of ammonia nitrogen content in 3 strains of nitrogen-fixing bacteria. |
| 图选项 |
2.2 人工构建水稻秸秆降解菌群 实验筛选出19株纤维素分解菌与3株固氮菌,与菌株B组合,根据组合后分解水稻秸秆能力确定最佳菌株,结果如图 2所示。菌株B单独培养3、5、8 d秸秆减重率分别为29.9%、34.0%、50.9%。菌株B+D2组合培养3、5、8 d秸秆减重率分别为41.0%、47.9%、64.6%,说明菌株D2能够促进B分解秸秆能力的提升。菌株B+D2+W1混合培养3、5、8 d秸秆减重率分别为44.3%、52.4%、71.0%,分解秸秆的能力比B单独培养和B+D2组合培养均有显著提升,说明菌株W1能有效促进B+D2组合秸秆转化。与G1菌组合形成B+D2+W1+G1组合时,8 d时秸秆分解率为73.3%,说明菌株G1能促进B+D2+W1组合提升水稻秸秆降解效果,但是菌株G1的作用贡献度要小于菌株D2和W1。需要注意的是,B+D2+W1+G1组合中培养5 d时分解效果相对B+D2+W1组提升明显,水稻秸秆在5 d和8 d时的分解量更为接近图 2-D,说明G1菌的加入某种程度上缩短了秸秆分解的周期。
 |
| 图 2 人工组合菌群过程(A、B、C、D)和最终组合结果(E) Figure 2 The process of artificial combination of flora (A, B, C, D) and the final combination result (E). |
| 图选项 |
2.3 菌株分类地位确定鉴定 实验菌株B、D2、W1和G1进行16S rRNA提取、测序。将所得测序序列分别命名为HLB、HLD、HLW、HLG后上传至GenBank,获取登录号分别为MW131641、MW131625、MW131626、MW131624。所得序列利用BLAST程序进行比对,选择高同源性序列,用MEGA 5.2软件从GenBank数据库中调取相似性较高的相关菌株基因序列构建系统发育树,实验结果如图 3所示,实验菌株B、D2、W1和G1的近缘种分别是B. cereus、B. amyloliquefaciens、O. intermedium和B. licheniformis。
 |
| 图 3 木质纤维素组合B (A)、D2 (B)、W1 (C)、G1 (D)菌株系统发育分析树 Figure 3 Phylogenetic tree of B (A), D2 (B), W1 (C) and G1 (D). The GenBank accession numbers are given in parentheses. The number at each branch points is the percentage supported by bootstrap. Bar 0.2 at the bottom is the sequence divergence. |
| 图选项 |
2.4 菌种组合后纤维素酶活性与固氮效果探究 纤维素酶是秸秆类物质生物降解的重要条件,实验定期测定与木质纤维素分解相关的3种纤维素酶活。B菌株单独培养时AC、CB、CMC的酶活均在第4天达到最大,分别为51.98、43.71、50.86 U/mL。在第8天时,AC、CB、CMC酶活分别下降至28.20、23.87、29.05 U/mL。而组合B+D2+W1+G1的AC、CB、CMC酶活最高值分别为17.70、9.41、16.90 U/mL,8 d时AC、CB、CMC三种酶活稳定至15.03、6.38、14.98 U/mL。菌株B纤维素酶活性高,D2、W1、G1三株菌加入后,纤维素酶活明显被稀释减小,但并没有完全稀释消失,说明菌株D2、W1、G1的纤维素酶活较小,加入后纤维素酶活也没有出现协同增加现象,是其他方面的原因导致水稻秸秆降解率增加(图 4)。
 |
| 图 4 各组合培养时纤维素酶活变化情况 Figure 4 Changes of cellulase activity in each group during culture. A: AC enzyme activity; B: CB enzyme activity; C: CMC enzyme activity. |
| 图选项 |
氮源是微生物生长的重要营养物质,所以本实验向微生物群体中添加固氮菌G1并检测氨氮含量变化,以期通过增加培养基中的营养元素来增加水稻秸秆的分解效率。实验结果如图 5所示,B+D2+W1+G1组在0–4 d增加迅速,4–8 d与B菌株单独培养时并无太大区别。G1加入后,整个群体氨氮含量增加不明显,并没有像预期一样表现出明显的固氮能力,结合B+D2+W1+G1组在培养8 d时整体的水稻秸秆分解效果仅仅提升2.3%,提升效果小,所以,此微生物菌群对水稻秸秆的转化率高与系统内氨氮源含量多少无关。
 |
| 图 5 B+D2+W1+G1组与B单独培养氨氮含量变化情况 Figure 5 Changes of ammonia nitrogen content in B+D2+W1+G1 group and B alone culture. |
| 图选项 |
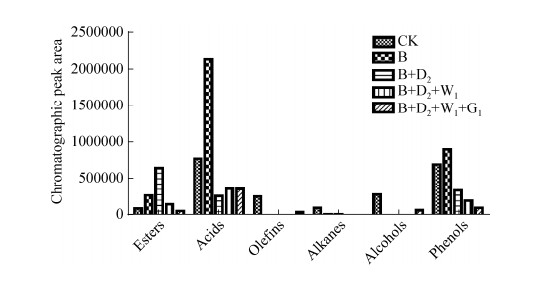
2.5 秸秆分解产物GC-MS分析结果 实验选取对照组(CK)、B菌株单独培养8 d、B+D2组合培养8 d、B+D2+W1组合培养8 d、B+D2+W1+G1组合培养8 d的秸秆分解产物进行GC-MS分析。经GC-MS检测发现,发酵产物主要为酯、酸、烯、烷、醇、酚6类。计算各菌剂组合与未接菌CK峰面积结果如图 6所示,B菌株单独培养8 d时,出现大量的酸、酚类物质。B+D2菌群比B菌株单独培养时,酸类减少87.4%,酚类减少61.9%。B+D2+W1组合比B+D2组合酚类减少41.1%。B+D2+W1+G1组合中酚类继续减少,减少程度不如B+D2和B+D2+W1组。结合每株菌加入后水稻秸秆降解效率的提升程度(图 2),表明稻秸秆降解效率的提升程度可能与系统内酸、酚类的含量负相关。
 |
| 图 6 不同组合中代谢产物成分比较 Figure 6 Comparison of components of metabolites in different combinations. |
| 图选项 |
进一步分析发现,B菌株单独培养时增加的酸类物质主要包括十五烷酸和正十六烷酸,酚类物质主要包括2, 4二叔丁基酚和6-叔丁基对甲酚。B+D2组合培养时减少的酸类物质主要为十五烷酸和正十六烷酸,减少的酚类物质主要为2, 4二叔丁基酚和6-叔丁基对甲酚,说明D2菌株具有减少系统中十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚含量的功能。B+D2+W1组合培养时进一步减少的酚类物质主要为6-叔丁基对甲酚,此外十五烷酸含量也进一步减少,说明W1菌株可能具有减少系统中6-叔丁基对甲酚和十五烷酸含量的功能。B+D2+W1+G1组合培养时6-叔丁基对甲酚再一次减少,说明G1菌株也可能具有减少系统中6-叔丁基对甲酚含量的功能,减少程度不如W1菌株(图 7)。
 |
| 图 7 含量变化明显的代谢产物的峰图 Figure 7 Peak area graph of metabolites with obvious changes in content. |
| 图选项 |
根据标准曲线(图 8)将菌株B单独培养时十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚(表 3)的峰面积换算成绝对含量。结果表明,菌株B单独培养8 d时十五烷酸含量为0.00586%,100 mL发酵液中纯质量为5.86 mg。正十六烷酸含量为0.00733%,100 mL发酵液中纯质量为7.33 mg。2, 4二叔丁基酚含量为0.00028%,100 mL发酵液中纯质量为0.28 mg。6-叔丁基对甲酚含量为0.00137%,100 mL发酵液中纯质量为1.37 mg。
 |
| 图 8 4种代谢产物的峰面积与含量标准曲线 Figure 8 Standard curve of peak area and content of 4 metabolites. A: Pentadecanoic acid; B: n-Hexadecanoic acid; C: 2, 4 Di-tert-butylphenol; D: 6-tert-butyl-p-cresol. |
| 图选项 |
表 3. 含量变化明显的代谢产物的峰面积 Table 3. Peak area of metabolites with obvious changes in content
| Rt | Name | Mf | Peak area | ||||
| CK | B | B+D2 | B+D2+W1 | B+D2+W1+G1 | |||
| 25.9 | Pentadecanoic acid | C15H30O2 | – | 484388 | 100158 | 59111 | – |
| 28.2 | n-Hexadecanoic acid | C16H32O2 | 72250 | 931146 | 157170 | 203518 | 196725 |
| 20.2 | Phenol, 2, 4-bis(1, 1-dimethylethyl)- | C14H22O | 29238 | 209155 | 53292 | 41179 | 37101 |
| 35.0 | Phenol, 2, 2'-methylenebis[6-(1, 1-dimethylethyl)-4-methyl- | C23H32O2 | 112151 | 709345 | 296976 | 164990 | 80914 |
| Rt: retention time; Mf: molecular formula; CK: control check; Phenol, 2, 4-bis(1, 1-dimethylethyl)-: 2, 4 Di-tert-butylphenol; Phenol, 2, 2'-methylenebis[6-(1, 1-dimethylethyl)-4-methyl-: 6-tert-butyl-p-cresol.–: None. | |||||||
表选项
2.6 分解产物抑制效果 根据绝对定量实验结果,将菌株B放入单独含不同浓度梯度的十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚4种物质的培养基中进行培养,观察菌株B对水稻秸秆降解效率的变化,结果如图 9所示。实验发现十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚4种物质对菌株B的水稻秸秆降解效率具有抑制作用,抑制作用大小:正十六烷酸>2, 4二叔丁基酚>十五烷酸>6-叔丁基对甲酚。培养基中加入与菌株B单独培养8 d时产生量相近的6 mg十五烷酸、8 mg正十六烷酸、3 mg 2, 4二叔丁基酚和2 mg 6-叔丁基对甲酚后,分别产生1.66%、17.53%、4.27%、3.35%的抑制效果。实验证明,十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚4种物质对菌株B的水稻秸秆降解效率确实具有抑制作用,菌种组合后水稻秸秆的分解效果提升的部分原因是外加菌株解除了部分抑制物的影响。
 |
| 图 9 不同代谢产物对分解水稻秸秆的抑制效果 Figure 9 Inhibitory effects of different metabolites on decomposition of rice straw. |
| 图选项 |
3 讨论 B. cereus分布广泛,常存在于腐烂的有机物、土壤、食物、淡水和海水以及无脊椎动物的肠道中[22],可生物合成多种水解酶,很早就已用于生物技术应用等领域[23]。B. cereus代谢会产生大量酸,Eder等[24]利用这一特性增加发酵氢气产量。Patel等[25]的研究也同样利用B. cereus产酸发酵。有机酸产生会使pH值降低,影响微生物生长和代谢[26]。同时,Low等[27]发现木质纤维素降解时主要由酚类单元中α-和β-醚键断裂以及非酚类单元中β-醚键断裂,所以水稻秸秆降解时系统内酚类物质会增加。酚类化合物在低浓度下会插入细菌膜的磷脂层,干扰脂酰链之间的范德华作用,使磷脂和膜的完整性受到破坏,对微生物具有毒性作用[28-30]。本实验中菌株B (B. cereus)单独培养降解水稻秸秆时,同样出现了大量酸类和酚类物质,这两类物质是阻碍水稻秸秆进一步生物降解的关键。B. amyloliquefaciens对不同有机酸具有趋化性反应[31]。Yuan等[32]研究发现,B. amyloliquefaciens生长过程中会吸收环境中的酚酸类物质,Hui等[33]也研究表示B. amyloliquefaciens产生的生物膜具有吸附亚硝酸盐的能力,且Mei等的研究表示B. amyloliquefaciens具有降解水稻秸秆的功能[34],这与本实验的D2 (B. amyloliquefaciens)菌株类似,当D2加入后,培养基酚酸类物质减少明显,秸秆降解效果增加。WAEL S.等从富含苯酚的活性污泥中分离出一株以苯酚为唯一碳源的O. intermedium,该菌株苯酚降解率较高[35]。本实验所用的菌株W1 (O. intermedium)能够减少系统内41.1%的酚类,对酚类物质也具有去除作用。
Eiteman等[10]利用一株大肠杆菌ZSC113 (不能消耗木糖)和另一株大肠杆菌ALS1008 (不能消耗葡萄糖)混合培养,获得了能高效发酵糖类的微生物群体。Szambelan等[12]将Kluyveromyces fragilis与Saccharomyces cerevisiae或者Zymomonas mobilis一起发酵,通过菌株间的协同作用使产氢量提高12%。本实验结果和机制与此相同,培养系统中各菌株通过种间互助和代谢互补来提高水稻秸秆的分解效率。秸秆降解过程中,构建的微生物组合中B菌株(B. cereus)与Aiya[36]筛选出的B. cereus JD 0404性状相似,能够分泌高活性的纤维素酶,这些酶可以水解木质纤维素并将其溶解为基本单体,以供其他微生物进一步代谢[37-38],培养基中每添加一次新菌种,三种纤维素酶活都会被不同程度稀释下降,菌株B具有较高酶活性是组合菌群降解水稻秸秆的基础,在整个体系中发挥着降解水稻秸秆的主要角色。菌株B在分解水稻秸秆后产生了大量的酸类(十五烷酸、正十六烷酸)、酚类(2, 4二叔丁基酚、6-叔丁基对甲酚)物质,菌株B不具有或不擅长降解这些酸、酚类物质的酶或代谢通路,所以此类产物逐渐积累致使菌株B的水稻秸秆降解率下降,添加菌株D2 (B. amyloliquefaciens)后发酵液中挥发性酸类减少87.4%,酚类减少61.9%,其中十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚等酚、酸类物质减少明显,秸秆降解效果得到第一次增加。菌株W1 (O. intermedium)能够减少系统内41.1%的酚类,对十五烷酸和6-叔丁基对甲酚去除率也比较明显,秸秆降解效果得到第二次增加,W1菌株的水稻秸秆降解效率和酚、酸类物质的去除性能都不如菌株D2,所以菌株W1加入后秸秆分解效率不如菌株D2加入时增加的多。菌株G1(B. licheniformis)接种至培养基后对整体的水稻秸秆分解效果提升不大,仅仅提升2.3%,这与菌株G1对酚、酸类物质去除能力小有关。但是菌株G1能提高中期(5 d)水稻秸秆的分解效率,具有一定缩短分解周期的作用,Liu等[39]研究中表示B. licheniformis能产生表面活性剂,对pH、温度和盐度具有良好的稳定性,表面活性剂存在时,疏水性物质在水中表面积增加,导致水溶性,从而增加微生物降解[40],菌株G1的具体作用功能可能与此有关,具体原因还待进一步研究。
4 结论 (1)成功构建出以菌株B (Bacillus cereus)、D2 (Bacillus amyloliquefaciens),W1 (Ochrobactrum intermedium)和G1 (Bacillus licheniformis)组成的能够在30 ℃培养8 d降解73.21%水稻秸秆的4菌微生物群体。
(2)微生物群体比单体对水稻秸秆转化效率高主要由于分解水稻秸秆时产生的代谢产物的反馈抑制,菌株B (B. cereus)单独接种到以水稻秸秆为唯一碳源的培养基时,发酵液中检测到大量的酸、酚类物质,其中酸类物质主要包括十五烷酸和正十六烷酸等,酚类物质主要包括2, 4二叔丁基酚和6-叔丁基对甲酚。高浓度的酸类和酚类物质对水稻秸秆降解过程起抑制作用,减少两类物质的含量可以提高菌株B的水稻秸秆分解效果。菌株D2 (B. amyloliquefaciens)能够有效减少系统内酸类和酚类的含量以提升水稻秸秆分解效果,对正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚减少作用明显。菌株W1 (O. intermedium)也能够有效减少系统内酸类和酚类的含量以提升水稻秸秆分解效果,对十五烷酸、正十六烷酸、2, 4二叔丁基酚和6-叔丁基对甲酚也具有减少作用,作用程度不如菌株D2。
(3)水稻秸秆成分复杂,单一菌种与多菌种组合成的菌剂相比,单一菌种降解效率不高,具有不同功能的菌种组合成复合菌剂可减少反馈抑制形成代谢互补,能有效提高水稻秸秆生物降解效果。
References
| [1] | Wang CF, Ma SC, Huang Y, Liu LY, Fan H, Deng Y. Characterization and microbial community shifts of rice strawdegrading microbial consortia. Acta Microbiologica Sinica, 2016, 56(12): 1856-1868. (in Chinese) 王春芳, 马诗淳, 黄艳, 刘来雁, 凡慧, 邓宇. 降解水稻秸秆的复合菌系及其微生物群落结构演替. 微生物学报, 2016, 56(12): 1856-1868. |
| [2] | Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiology and Molecular Biology Reviews, 1997, 61(2): 262-280. |
| [3] | Wolfaardt GM, Lawrence JR, Robarts RD, Caldwell DE. The role of interactions, sessile growth and nutrient amendments on the degradative efficiency of a microbial consortium. Canadian Journal of Microbiology, 1994, 40(5): 331-340. DOI:10.1139/m94-055 |
| [4] | Mikesková H, Novotny ?, Svobodová K. Interspecific interactions in mixed microbial cultures in a biodegradation perspective. Applied Microbiology and Biotechnology, 2012, 95(4): 861-870. DOI:10.1007/s00253-012-4234-6 |
| [5] | Abreu NA, Taga ME. Decoding molecular interactions in microbial communities. FEMS Microbiology Reviews, 2016, 40(5): 648-663. DOI:10.1093/femsre/fuw019 |
| [6] | Ghosh S, Chowdhury R, Bhattacharya P. Mixed consortia in bioprocesses: role of microbial interactions. Applied Microbiology and Biotechnology, 2016, 100(10): 4283-4295. DOI:10.1007/s00253-016-7448-1 |
| [7] | Weimer PJ, Zeikus JG. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Applied and Environmental Microbiology, 1977, 33(2): 289-297. DOI:10.1128/aem.33.2.289-297.1977 |
| [8] | Pohlschroeder M, Leschine SB, Canale-Parola E. Spirochaeta caldaria sp. nov., a thermophilic bacterium that enhances cellulose degradation by Clostridium thermocellum. Archives of Microbiology, 1994, 161(1): 17-24. |
| [9] | Ng TK, Ben-Bassat A, Zeikus JG. Ethanol production by thermophilic bacteria: fermentation of cellulosic substrates by cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Applied and Environmental Microbiology, 1981, 41(6): 1337-1343. DOI:10.1128/aem.41.6.1337-1343.1981 |
| [10] | Eiteman MA, Lee SA, Altman E. A co-fermentation strategy to consume sugar mixtures effectively. Journal of Biological Engineering, 2008, 2: 3. DOI:10.1186/1754-1611-2-3 |
| [11] | Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiology and Molecular Biology Reviews, 2002, 66(3): 506-577. DOI:10.1128/MMBR.66.3.506-577.2002 |
| [12] | Szambelan K, Nowak J, Czarnecki Z. Use of Zymomonas mobilis and Saccharomyces cerevisiae mixed with Kluyveromyces fragilis for improved ethanol production from Jerusalem artichoke tubers. Biotechnology Letters, 2004, 26(10): 845-848. DOI:10.1023/B:BILE.0000025889.25364.4b |
| [13] | Jiménez DJ, Dini-Andreote F, DeAngelis KM, Singer SW, Salles JF, van Elsas JD. Ecological insights into the dynamics of plant biomass-degrading microbial consortia. Trends in Microbiology, 2017, 25(10): 788-796. DOI:10.1016/j.tim.2017.05.012 |
| [14] | Brenner K, You LC, Arnold FH. Engineering microbial consortia: a new frontier in synthetic biology. Trends in Biotechnology, 2008, 26(9): 483-489. DOI:10.1016/j.tibtech.2008.05.004 |
| [15] | Mee MT, Wang HH. Engineering ecosystems and synthetic ecologies. Molecular BioSystems, 2012, 8(10): 2470. DOI:10.1039/c2mb25133g |
| [16] | Evans R, Alessi AM, Bird S, Mcqueen-Mason SJ, Bruce NC, Brockhurst MA. Defining the functional traits that drive bacterial decomposer community productivity. The ISME Journal, 2017, 11(7): 1680-1687. DOI:10.1038/ismej.2017.22 |
| [17] | Wang AJ, Gao LF, Ren NQ, Xu JF, Liu C, Lee DJ. Enrichment strategy to select functional consortium from mixed cultures: Consortium from rumen liquor for simultaneous cellulose degradation and hydrogen production. International Journal of Hydrogen Energy, 2010, 35(24): 13413-13418. DOI:10.1016/j.ijhydene.2009.11.117 |
| [18] | Wang WD, Wang XF, Liu CL, Li YH, Lü YC, Cui ZJ. Productions analyses and pH dynamics during rice straw degradation by the lignocellulose degradation bacteria system WSC-6. Environmental Science, 2008, 29(1): 219-224. (in Chinese) 王伟东, 王小芬, 刘长莉, 李玉花, 吕育财, 崔宗均. 木质纤维素分解菌复合系WSC-6分解稻秆过程中的产物及pH动态. 环境科学, 2008, 29(1): 219-224. DOI:10.3321/j.issn:0250-3301.2008.01.037 |
| [19] | Sadana JC, Patil RV. Synergism between enzymes of Sclerotium rolfsii involved in the solubilization of crystalline cellulose. Carbohydrate Research, 1985, 140(1): 111-120. DOI:10.1016/0008-6215(85)85054-0 |
| [20] | Rouches E, Dignac MF, Zhou SM, Carrere H. Pyrolysis-GC-MS to assess the fungal pretreatment efficiency for wheat straw anaerobic digestion. Journal of Analytical and Applied Pyrolysis, 2017, 123: 409-418. DOI:10.1016/j.jaap.2016.10.012 |
| [21] | Chen DY, Wang Y, Liu YX, Cen KH, Cao XB, Ma ZQ, Li YJ. Comparative study on the pyrolysis behaviors of rice straw under different washing pretreatments of water, acid solution, and aqueous phase bio-oil by using TG-FTIR and Py-GC/MS. Fuel, 2019, 252: 1-9. DOI:10.1016/j.fuel.2019.04.086 |
| [22] | Bottone EJ. Bacillus cereus, a volatile human pathogen. Clinical Microbiology Reviews, 2010, 23(2): 382-398. DOI:10.1128/CMR.00073-09 |
| [23] | Laba W, Kope? W, Chor??yk D, Kancelista A, Piegza M, Malik K. Biodegradation of pretreated pig bristles by Bacillus cereus B5esz. International Biodeterioration & Biodegradation, 2015, 100: 116-123. |
| [24] | Eder AS, Magrini FE, Spengler A, da Silva JT, Beal LL, Paesi S. Comparison of hydrogen and volatile fatty acid production by Bacillus cereus, Enterococcus faecalis and Enterobacter aerogenes singly, in co-cultures or in the bioaugmentation of microbial consortium from sugarcane vinasse. Environmental Technology & Innovation, 2020, 18: 100638. |
| [25] | Patel SKS, Singh M, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of Bacillus spp. from glucose in two stage system. Indian Journal of Microbiology, 2011, 51(4): 418-423. DOI:10.1007/s12088-011-0236-9 |
| [26] | Nakasaki K, Araya S, Mimoto H. Inoculation of Pichia kudriavzevii RB1 degrades the organic acids present in raw compost material and accelerates composting. Bioresource Technology, 2013, 144: 521-528. DOI:10.1016/j.biortech.2013.07.005 |
| [27] | Low JC, Halis R, Shah UKM, Paridah MT, Abood F, Tuhaila T, Danial MI, Lakarim L, Razali N. Enhancing enzymatic digestibility of alkaline pretreated banana pseudostem for sugar production. BioResources, 2015, 10(1): 1213-1223. |
| [28] | Kumar H, Mohanty K. Kinetic modelling of phenol biodegradation by mixed microbial culture in static batch mode. Asian Journal of Water, Environment and Pollution, 2012, 9(3): 19-24. |
| [29] | Liu YJ, Zhang AN, Wang XC. Biodegradation of phenol by using free and immobilized cells of Acinetobacter sp. XA05 and Sphingomonas sp. FG03. Biochemical Engineering Journal, 2009, 44(2/3): 187-192. |
| [30] | Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. International Journal of Food Microbiology, 2004, 94(3): 223-253. DOI:10.1016/j.ijfoodmicro.2004.03.022 |
| [31] | Tan SY, Yang CL, Mei XL, Shen SY, Raza W, Shen QR, Xu YC. The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Applied Soil Ecology, 2013, 64: 15-22. DOI:10.1016/j.apsoil.2012.10.011 |
| [32] | Yuan J, Wu YC, Zhao ML, Wen T, Huang QW, Shen QR. Effect of phenolic acids from banana root exudates on root colonization and pathogen suppressive properties of Bacillus amyloliquefaciens NJN-6. Biological Control, 2018, 125: 131-137. DOI:10.1016/j.biocontrol.2018.05.016 |
| [33] | Hui C, Guo XX, Sun PF, Khan RA, Zhang QC, Liang YC, Zhao YH. Removal of nitrite from aqueous solution by Bacillus amyloliquefaciens biofilm adsorption. Bioresource Technology, 2018, 248: 146-152. DOI:10.1016/j.biortech.2017.06.176 |
| [34] | Mei JF, Shen XB, Gang LP, Xu HJ, Wu FF, Sheng LQ. A novel lignin degradation bacteria-Bacillus amyloliquefaciens SL-7 used to degrade straw lignin efficiently. Bioresource Technology, 2020, 310: 123445. DOI:10.1016/j.biortech.2020.123445 |
| [35] | El-Sayed WS, Ibrahim MK, Abu-Shady M, El-Beih F, Ohmura N, Saiki H, Ando A. Isolation and identification of a novel strain of the genus Ochrobactrum with phenol-degrading activity. Journal of Bioscience and Bioengineering, 2003, 96(3): 310-312. DOI:10.1016/S1389-1723(03)80200-1 |
| [36] | Chantarasiri A. Aquatic Bacillus cereus JD0404 isolated from the muddy sediments of mangrove swamps in Thailand and characterization of its cellulolytic activity. The Egyptian Journal of Aquatic Research, 2015, 41(3): 257-264. DOI:10.1016/j.ejar.2015.08.003 |
| [37] | López-González JA, López MJ, Vargas-García MC, Suárez-Estrella F, Jurado M, Moreno J. Tracking organic matter and microbiota dynamics during the stages of lignocellulosic waste composting. Bioresource Technology, 2013, 146: 574-584. DOI:10.1016/j.biortech.2013.07.122 |
| [38] | Zhao Y, Zhao Y, Zhang ZC, Wei YQ, Wang H, Lu Q, Li YJ, Wei ZM. Effect of thermo-tolerant actinomycetes inoculation on cellulose degradation and the formation of humic substances during composting. Waste Management, 2017, 68: 64-73. DOI:10.1016/j.wasman.2017.06.022 |
| [39] | Liu BQ, Liu JP, Ju MT, Li XJ, Yu QL. Purification and characterization of biosurfactant produced by Bacillus licheniformis Y-1 and its application in remediation of petroleum contaminated soil. Marine Pollution Bulletin, 2016, 107(1): 46-51. DOI:10.1016/j.marpolbul.2016.04.025 |
| [40] | Karanth NGK, Deo PG, Veenanadig NK. Microbial production of biosurfactants and their importance. Current Science, 1999, 77(1): 116-126. |
