李静, 张宝刚

 , 刘青松, 韩亚伟
, 刘青松, 韩亚伟 中国地质大学(北京)水资源与环境学院, 地下水循环与环境演化教育部重点实验室, 北京 100083
收稿日期:2021-03-20;修回日期:2021-05-17;网络出版日期:2021-05-26
基金项目:国家自然科学基金(42022055)
作者简介:张宝刚,山东淄博人,教授,博导,国家优秀青年基金获得者,国家生态环境保护专业技术青年拔尖人才,美国科罗拉多大学访问****。一直致力于重金属环境生物地球化学过程及高效修复研究,涉及微生物、地质学、电化学、光催化及能源材料领域。以第一或通讯作者发表SCI论文70余篇,包括环境、地球科学领域Top期刊Environmental Science & Technology、Geochimica et Cosmochimica Acta、Water Reseach等。主持国家自然科学基金5项、省部级项目9项、横向项目10余项。以第一发明人授权发明专利5项。入选“北京市科技新星计划”,获中国地质学会第十六届青年地质科技奖(银锤奖)、第二届中国环境科学学会青年科学家奖(优秀奖).
*通信作者:张宝刚, Tel: +86-10-82322281;Fax: +86-82321081; E-mail: baogangzhang@cugb.edu.cn.
摘要:厌氧条件下,微生物可以通过厌氧代谢产生甲烷(CH4),由此衍生的厌氧消化技术可实现能源的回收利用。产CH4的关键步骤是刺激发酵细菌和产甲烷古菌之间的有效电子转移,电活性微生物可以取代传统的氢/甲酸盐实现直接种间电子传递,其电子传递效率更高。添加导电材料可以促进直接种间电子传递并提高CH4产率,是一种更有效的强化电子传递方式。本文在梳理直接种间电子传递发展和机理的基础上,综述了常见的促进直接种间电子传递的碳基和铁基导电材料,对其结构特征、电子传递机理、强化产CH4和中间产物消耗等方面进行了系统总结。旨在为导电材料促进直接种间电子传递的研究提供参考,并探讨了未来可能的研究方向。
关键词:厌氧消化甲烷直接种间电子传递导电材料
Research progress on enhancement of methane production through direct interspecific electron transfer by conductive materials
Li Jing, Zhang Baogang

 , Liu Qingsong, Han Yawei
, Liu Qingsong, Han Yawei Key Laboratory of Groundwater Circulation and Environmental Evolution, Ministry of Education, School of Water Resources and Environment, China University of Geosciences Beijing, Beijing 100083, China
Received: 20 March 2021; Revised: 17 May 2021; Published online: 26 May 2021
*Corresponding author: Baogang Zhang, Tel: +86-10-82322281; Fax: +86-82321081; E-mail: baogangzhang@cugb.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (42022055)
Abstract: Under anaerobic conditions, microorganisms produce methane (CH4) through anaerobic metabolism. The derived anaerobic digestion technology realizes energy recovery. The key step of CH4 production is to stimulate the effective electron transfer between fermentation bacteria and methanogens. Electroactive microorganisms can replace the traditional hydrogen/formate to achieve direct interspecific electron transfer, with higher electron transfer efficiency. The addition of conductive materials promotes direct interspecific electron transfer and increase the yield of CH4, which is a more effective way to enhance the electron transfer. Based on the development and mechanism of direct interspecific electron transfer, carbon-based and iron-based conductive materials that promote direct interspecific electron transfer are comprehensively reviewed. The structural characteristics, electron transfer mechanism, enhanced CH4 production and intermediate consumption by these materials are systematically summarized. This review aims to provides reference for the research of conductive materials promoting direct interspecific electron transfer, and to explore the possible research direction in future.
Keywords: anaerobic digestionmethanedirect interspecific electron transferconductive material
厌氧产甲烷(CH4)是地质微生物参与的重要地球化学过程,也是自然界碳循环的重要环节[1]。以产CH4为核心的厌氧消化(anaerobic digestion,AD)可以生产清洁的可再生能源,逐渐成为能源领域研究和应用的热点[2]。AD包括3个阶段:水解、产酸和产CH4[3]。在水解酸化阶段,在水解酶的作用下,有机物大分子转化为短链脂肪酸和醇等中间体。产酸阶段产生大量H2使分压增加(ΔH>0),产CH4反应在热力学上不能自发进行,只有当同营养微生物(两种微生物均需要对方为自身的生长提供必要的代谢支持[4])将H2去除时才可行。由于产甲烷古菌代谢缓慢,对环境敏感,挥发性脂肪酸(volatile fatty acids,VFAs)的积累和H2分压升高会抑制产CH4过程,造成AD效率低和稳定性差[5]。以H2/甲酸盐作为电子载体,细菌和产甲烷古菌之间可以发生营养代谢,进行间接种间电子传递(mediated interspecies electron transfer,MIET)[6],从而消除产CH4过程中的障碍。最近研究表明,除了种间H2/甲酸盐转移,一些电化学活性细菌和产甲烷古菌之间还可以发生直接电子传递[7-8]。这些细菌通过鞭毛上分布的导电菌毛和c型细胞色素(c-type cytochrome,c-Cyts)发挥作用,将电子转移到产甲烷古菌[9-11],这种电子转移机制被称作直接种间电子传递(direct interspecies electron transfer,DIET)。这种电子传递方式不需要添加额外的能量产生H2作为电子穿梭体就可以促进CH4的高效产生[12-14]。
DIET过程的强化,可以进一步提升CH4的产生效率,逐渐成为AD领域研究的热点。近年来研究表明,导电材料(conductive material,CM)可以加强DIET作用,其中碳基与铁基CM研究最为广泛,例如石墨毡[15]、活性炭[16]、生物炭[17]、水热炭[18]、碳布[19]、碳纳米管[20]、磁铁矿[21]、纳米零价铁[22]的添加可以刺激DIET的发生,改变微生物群落,促进功能微生物富集,加快VFAs的消耗,从而提高CH4产生率[23-24]。
本文在归纳DIET发展与机理的基础上,梳理可强化DIET的碳基和铁基CM及其对AD过程的影响,从而归纳出DIET的未来前景与发展方向。
1 DIET发展与机理 首次发现DIET是在Geobacter metallireducens (金属还原地杆菌)和Geobacter sulfreducens (硫还原地杆菌)的共培养体系中,以富马酸为电子受体,乙醇为电子供体,发现微生物形成导电聚集物,且聚集物形成一种突变,该突变能够增强c-Cyts的生成。长而灵活的导电菌毛为微生物间的电接触提供通道,并且具有足够的导电性以满足微生物的电子传递需求[25]。敲除两细菌的基因omcS (一种多血红素c-Cyts基因)或pilR(结构性菌毛蛋白基因)均无法进行电子互营,表明c-Cyts和菌毛在DIET中都发挥重要作用[26]。由此揭开了DIET研究的序幕。
微生物中有各种各样的氧化还原蛋白,c-Cyts参与氧化还原介导的与呼吸有关的电子传递反应,且不同的电子传递模式下发挥作用的c-Cyts种类各不相同(图 1)。参与DIET最常见的Geobacter (地杆菌)中包括OmcB、OmcC、OmcS和OmcE多种蛋白质[27]。Geobacter sulfreducens电子转移过程的关键途径是由各种c-Cyts (OmcB和OmcC)介导。Geobacter sulfreducens还原细胞外金属氧化物时,多血型c-Cyts起关键作用,敲掉omcS和omcE任一基因都会降低Mn(Ⅳ)和Fe(Ⅲ)氧化物的还原能力[28]。细胞色素中有专门的功能蛋白可以通过电子穿梭将电子送到更远的电子受体。如在硫还原地杆菌中,膜结合蛋白NADH-脱氢酶通过细胞色素链穿过膜[29];在Shewanella oneidensis (奥奈达希瓦氏菌)中的电子转移是由甲酸脱氢酶起作用[30-31]。参与较薄生物膜的胞外电子传递的主要蛋白质为OmcS和OmcE,较厚生物膜的胞外电子传递的主要为菌毛蛋白(PilA)[32]。
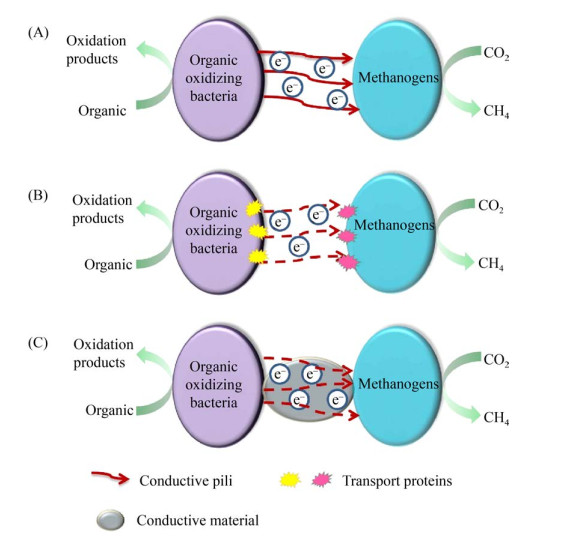 |
| 图 1 DIET发生的三种途径(该图修改自文献[33]) Figure 1 Three pathways of DIET[33]. A: conductive pili; B: transport proteins; C: conductive material. |
| 图选项 |
菌毛通常被称为微生物纳米线,是一种具有类似金属导电性的蛋白质细丝,在适当条件下可以促进远距离的电子传递,还可以进行微生物之间的DIET[34]。这些纳米线参与了土壤和沉积物中碳和矿物的循环、生物修复、有机物向CH4或电能的转化等一系列氧化还原过程[35]。富含PilA的Pseudomonas (假单胞菌属)和Desulfurella (硫还原菌属)能够直接利用复杂有机物作为电子供体,进行DIET介导的同营养代谢[36]。在Geobacter和Methanosaeta (甲烷鬓毛菌)或Methanosarcina (甲烷八叠球菌)的同营养菌共培养中,发现了导电菌毛介导的DIET产CH4作用[37]。然而,最近研究表明,导电菌毛并不是由PilA构成,而是由OmcS聚合链组成。PilA的作用是调节OmcS纳米线以及其他多血红素细胞色素的分泌。PilA的过度表达伴随着OmcS细丝的过度产生,并将OmcS分泌到细胞外环境中[38]。因此,关于不同细菌纳米线的组成及功能,还需要进一步研究。
AD是一种经济有效的处理有机废物的方法,可以提高消化质量和CH4产量。但是传统的AD面临着一些瓶颈:启动时间长;有机物水解率低,有毒物质和VFAs的积累会抑制CH4的生成;H2的积累抑制产乙酸细菌的氧化辅酶[39]。DIET是微生物同营养氧化代谢中一种有效的途径,可以提高AD的速率和效率。外生电细菌可以通过c-Cyts或导电菌毛将电子转移到电子受体细菌[40]。AD与DIET的结合可克服上述存在的问题,改善酸的积累并提高CH4产率。CM的添加可以替代c-Cyts和导电菌毛成为电子连接装置,为细胞节省能量,还可以实现远距离电子传输,加速DIET现象[15]。CM会促进氧化还原蛋白如c-Cyts和黄素蛋白的表达[41-42]。添加CM的DIET可以显著提高底物的降解和CH4产率,抑制VFAs的积累,并且可缩短系统启动时间和提高AD系统的稳定性[43]。因此,CM强化DIET过程促进产CH4作用引起越来越多的关注与研究。
2 CM介导DIET促进产CH4原理及应用 DIET过程可以通过CM刺激并发生强化,CM在微生物之间形成导电管道实现电子传递。CM可以起到与c-Cyts和菌毛相似的作用,这方面研究主要集中在碳基与铁基CM。碳基CM为微生物提供更大的反应表面积,有利于微生物的附着。还可以利用自身的大孔径吸附有毒化合物,以避免干扰产CH4过程[44]。碳基CM的导电性比菌毛更高,在Geobacter metallireducens与Methanosarcina barkeri (巴氏甲烷八叠球菌)的共培养中,可以代替菌毛进行电子传递[45],比菌毛具有更好的产CH4性能。铁基CM在缺乏菌毛蛋白的Geobacter中具有双重作用,既可以刺激关键酶的分泌,又可以促进DIET相关蛋白的表达[46]。结晶的纳米级矿物可以使微生物的菌毛延伸并且使微生物集合体具有导电性,从而与细胞外电子受体建立联系[47]。在导电磁铁矿纳米颗粒和半导电赤铁矿纳米颗粒的存在下,一些c-Cyts基因例如omcJ、pgcA和omcK的表达会上调[48]。由于生物膜中很容易嵌入纳米级矿物,所以它们构成的紧密界面系统可以提供更多的活性位点,有利于微生物的接触。因此,碳基和铁基CM在DIET促进产CH4的研究中日益兴起。
2.1 碳基CM 碳基CM具有高比表面积和优异的导电性,这些特性对于促进DIET提高甲烷产率至关重要[49-50]。碳基CM的多孔结构和高比表面积可为微生物提供更大的附着空间和合适的生长环境,促进微生物的生长代谢,有利于电子供体微生物、充当电子转移站的CM和电子受体微生物之间更好地接触,从而发生DIET以促进CH4产生[51-52]。CM的导电性和表面氧化还原官能团也与DIET的效率密切相关,表面氧化还原官能团可显著提高CH4产生速率[53]。醌和对苯二酚与CM的氧化还原官能团有关,它们能够提供和接受电子使CM具有氧化还原活性。具有氧化还原活性的CM可以作为电子穿梭体促进电子供体微生物和电子受体微生物之间的DIET[17]。碳基CM存在一定缺陷,比如:生物炭在低热解温度(< 350 ℃)下制备具有较丰富的官能团,但导电性较差;在较高热解温度(> 700 ℃)下电导率有所提升,但表面官能团有所减少。因此,最近很多研究采用对生物炭改性的方法来提高其各种性能,例如负载硫改性纳米零价铁[54]、铁锰改性生物炭复合材料[55]、负载Fe3O4[56]和芬顿污泥衍生的含磁铁矿生物炭[57]。金属材料与碳基CM的结合不仅有利于碳基CM的分离和回收,还改善了不同微生物之间的协作关系[53]。然而,碳基CM的制备过程,大多需要高温高压的条件,操作较为复杂,还有毒性有机试剂的应用,未来需探索更为简便有效、节能环保的制备方法。
碳基CM的添加可显著促进产CH4过程。图 2比较了文献报道的不同碳基CM在混菌中温(35-38 ℃)厌氧反应器中不同剂量下的CH4产率。碳基CM使CH4产率提高了0.26%-890.76%[16, 20, 24, 58-75]。颗粒活性炭和生物炭研究较多[76-91]。CH4产率整体上随CM用量的增加而增加,其中石墨烯在此方面的规律性最为明显。其他碳基CM并未观察到明显的规律,可能是由于材料制备工艺的差别。向厌氧消化池中添加石墨烯可显著提高CH4产量,并增加Geobacter和Methanosarcina等电活性微生物的数量[24]。Igarashi等通过同位素标记实验检测了共培养的碳通量,证明了DIET依赖的CO2还原是石墨烯促进CH4化的原因,亲水性胺官能化的石墨烯实现最高的CH4生成率,证明表面亲水性与DIET促进效率呈正相关[92]。添加生物炭可缩短产CH4的滞后时间和增加最大CH4产率,生物炭表面存在的醌和氢醌官能团可以促进电活性微生物之间的电子传递[74]。向AD系统添加适量MnFe2O4改性生物炭能够建立起DIET,提高系统的缓冲能力,促进污泥中可溶性蛋白质和碳水化合物的降解,从而提高CH4产量[55]。在400 ℃下热解芬顿污泥可获得含磁铁矿的生物炭,具有高的电容量和电导率,磁铁矿充当电子管道,促进营养细菌和产甲烷古菌之间的DIET,从而提高CH4产率[57]。污泥衍生的水热炭在10 g/L剂量下对CH4产生速率的促进效果最佳,且不控制pH条件下最大CH4产率更高,说明污泥衍生的水热炭在抑制条件下(如低pH)更能够促进CH4的生成[58]。有碳布存在的AD系统中,种间电子交换的主要工作模式是DIET,因此形成稳定的产CH4作用。互营微生物都倾向于附着在碳布上进行种间电子交换,可以节省细胞能量和产生更少的细胞外生物电连接[12]。单壁碳纳米管形成电子传输纳米线通过DIET连接乙酸盐氧化和产甲烷古菌之间的电子传递,类似导电菌毛的纳米线,可以使污泥获得更高的电导率,从而对电子传输产生积极影响[93]。未来,除继续探究碳基CM对产CH4的促进作用外,还需关注其使用后的回收与再利用。
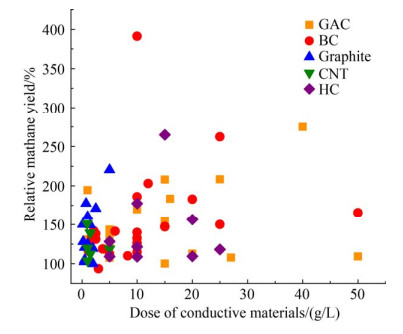 |
| 图 2 不同碳基CM的产CH4反应器性能的比较[16, 20, 24, 58-91] Figure 2 Performance comparison of CH4 reactors with different carbon based CM[16, 20, 24, 58-91]. |
| 图选项 |
碳基CM可提高VFAs的消耗速率,减少中间代谢产物的积累。石墨毡可以替代导电菌毛充当VFAs氧化菌与产甲烷古菌在同营养代谢过程中的导电物质,在高H2分压下利用DIET作用机制确保丙酸和丁酸的转化[15]。颗粒活性炭修饰的厌氧消化池中乙酸的最大浓度仅为对照组的一半,并且浓度会逐渐下降直到完全消失[92]。适量的柑橘皮生物炭可以缓解高有机负荷导致的系统酸化,其结构中的碱性官能团能有效地中和部分VFAs,促进VFAs向CH4的转化并加速pH恢复。柑橘皮生物炭刺激微生物形成DIET加速H2的消耗,使丙酸的降解反应热力学平衡右移[74]。柑橘皮生物炭的加入不仅降低总VFAs中丙酸的含量,减轻丙酸对产CH4的抑制作用,还可降低本应该升高的丙酸的降解产物乙酸的含量[60]。高浓度的氨氮会对AD系统产生毒性,MnFe2O4改性生物炭的较强吸附力能有效缓解氨抑制,减少滞后时间并提高系统的稳定性,低浓度的MnFe2O4改性生物炭可以有效地促进VFAs的转化效率和提高系统的缓冲能力,微量元素Mn、Fe的补充还可以增强底物的水解酸化过程[55]。由此可见,碳基CM可改善产甲烷古菌的生存环境,避免对其生长造成抑制。
2.2 铁基CM 铁基CM在AD过程中应用十分广泛,用于AD系统中促进DIET的铁基材料有零价铁[94-95]、磁性纳米颗粒[95]、硫改性纳米零价铁[96]、磁铁矿[97-98]等,分别用于氨氮胁迫条件下废活性污泥、有机污染物的厌氧还原、污泥与餐厨垃圾厌氧共消化和上流厌氧污泥床反应器中。零价铁已应用于地下水净化、污水处理和土壤修复,是一种低成本且寿命长的导电材料[99]。零价铁可以降低系统的氧化还原电位,为厌氧微生物提供更适合生长的环境[43, 100-101]。不过在运行中,零价铁表面可能会产生铁(氢)氧化物而抑制活性[102]。在表面涂覆硫化物形成硫改性纳米零价铁可以提高其活性[103],同时,硫改性纳米零价铁比纳米零价铁的比表面积更大、反应活性更高、生物毒性更低[104]。铁氧化物Fe3O4具有理想电势,可以作为电子通道刺激DIET促进CH4的产生[105]。磁铁矿是土壤和沉积物中一种导电矿物,很多研究用其建立DIET以促进产CH4作用[106]。铁基CM可通过绿色环保的生物方法制备,部分铁基CM也来自自然界,因此可以实现规模化应用。
铁基CM对AD产CH4有明显的促进作用。图 3比较了文献中的不同铁基CM在混菌中温(35-38 ℃)厌氧反应器中不同剂量下的CH4产率。添加铁基CM使CH4产率提高了1.13%-254.74%[46, 57, 95-96, 105, 107-110],铁基CM的种类和剂量影响CH4产率[111-129]。CH4产率随着磁铁矿、硫改性纳米零价铁、零价铁和赤泥投加量的增加而逐渐增大,但当赤铁矿用量超过16 g/L时,对CH4产率不再有促进作用。对比零价铁、磁性纳米颗粒和颗粒活性炭在氨胁迫条件下的表现,发现添加零价铁的系统产CH4性能最好,其次是磁性纳米颗粒[60]。零价铁通过抑制氨促进了微生物的抗毒活性,使CH4生成率降低最少,滞后时间最短,污泥减量率最高。零价铁可能取代促进DIET的醌氧化还原酶(EtfAB酶)的作用[95],可以通过刺激酶活性、产生额外的H2和减少酸的积累,从而提高CH4产率[102]。与零价铁增强AD的机制不同,硫改性纳米零价铁可以提高底物的利用率,并且通过更有效的电子传递促进H2向CH4的转化[96]。Fe3O4会与产甲烷古菌竞争电子,从而轻微抑制产CH4作用。但Fe3O4会强化水解-酸化过程,生成更多的CO2/H2转化成CH4。增强作用高于抑制作用,所以最终表现为促进CH4生成[97]。在富含硫酸盐废水的上流式厌氧污泥反应器中,添加磁铁矿的CH4产率在第40天达到157 mL/d,不添加磁铁矿的反应器中CH4产率稳定在48 mL/d左右,只有前者的1/3。进水硫酸盐浓度增加后,添加磁铁矿比未添加磁铁矿的平均CH4生产率高3-10倍,差距进一步增大[98]。然而,有研究表明Fe(Ⅲ)会抑制AD中CH4的生成[130],因此铁基CM的长期效应,值得进一步评估。
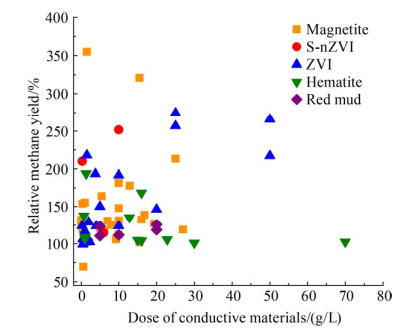 |
| 图 3 不同铁基CM的产CH4反应器性能的比较[46, 57, 95-96, 105, 107-129] Figure 3 Performance comparison of CH4 production reactors with different iron-based CM[46, 57, 95-96, 105, 107-129]. |
| 图选项 |
铁基CM可以促进中间产物的快速消耗。在污泥的厌氧发酵池中添加20 g/L零价铁,可以促进短链脂肪酸向中链脂肪酸和长链醇的转化。零价铁剂量与促进作用呈正相关,最高剂量(20 g/L)零价铁仍然具有促进作用。零价铁的存在还可以促进废活性污泥的增溶过程,以及多糖、蛋白质和溶解性化学需氧量的释放和氨基酸的酸化[95]。在氨胁迫条件下,零价铁对VFAs的降解作用最好[94]。而没有氨胁迫条件下,Fe3O4对VFAs的降解效果优于零价铁[131]。VFAs快速被消耗后,环境条件得以改善,可有效提高产甲烷古菌的活性。
3 CM影响微生物群落结构 CM的添加会使微生物群落结构发生变化[132],根据16S rRNA基因高通量测序的门级鉴定分析显示,不添加CM的样本中以Firmicutes (厚壁菌门)、Euryarchaeota (广古菌门)、Bacteroidetes (拟杆菌门)、Chloroflexi (绿弯菌门)、Aminicenantes (氨酸菌门)、Proteobacteria (变形菌门)和Synergistetes (互养菌门)为主[53]。CM的添加使Firmicutes的相对丰度显著增加,该菌属含有能够降解VFAs的同营养细菌,可加速乙酸盐的产生。Firmicutes中最丰富的Clostridium (梭状芽孢杆菌)可提高VFAs的水解率[133]。属于Firmicutes的Trichococcus (毛球菌属)的相对丰度也有所增加,其可将葡萄糖降解为乳酸、乙酸和乙醇,但无直接证据表明DIET的发生[53]。
在CM刺激下富集的电子供体微生物如Trichococcus[94]、Clostridium[133]、Candidatus (念珠菌)[133]、Syntrophomonas[95]和Desulfovibrio (脱硫弧菌)[15]等,其作用都是通过降解VFAs或与产甲烷古菌形成同营养代谢以促进CH4产生,但无直接证据表明其参与了DIET。因此这些细菌仅仅是DIET的潜在功能微生物,并不能完全证明DIET的发生。Geobacter是至今所发现的唯一可以在不同微生物之间发生DIET的细菌[26]。Geobacter是在DIET中最丰富和活跃的同营养细菌,在微生物群落中担任主要角色,成为DIET发生的“标志”。但表 1和表 2显示,在CM刺激DIET的反应体系中有接近半数研究并未检测到Geobacter或者丰度很低(5%以下),原因可能是DIET并没有真正发生,CH4增加是由于CM发挥其他作用所导致,如增加微生物附着、刺激有机物降解;也可能是因为存在尚未被证实的电活性细菌,难以证实已发生的DIET[134]。由此可见,能进行DIET的微生物不仅限于Geobacter,DIET功能微生物资源值得深入挖掘,尤其在添加CM的体系中,DIET过程进行得更剧烈,可能会富集更多的DIET功能微生物,但如何证明潜在功能物种的DIET能力是未来研究的方向。
表 1. 产CH4体系中碳基CM的作用识别及富集微生物种类 Table 1. Functions of carbon-based CM in CH4 production system with enriched functional microbes
| Type | Dosage | Inoculum | Substrate | Identification techniques | Functions | Enriched microbes | References |
| HC | 10.0 g/L | UASB reactor | Glucose | qPCR 16S rRNA | Improve CH4 yield Reduce lag time Alleviate ammonia inhibition | Firmicutes Methanolinea | [53] |
| BC | 15.0 g/L | AD system | Phenol | FT-IR EC MER/MEO | Improve microbial activity Improve CH4 yield | Geobacter Syntrophorhabdus Methanobacterium | [74] |
| BC | 10.0 g/L | AD system | Food waste/Waste activated sludge | 16S rRNA | Enhance pollutant degradation | Geobacter Sphaerochaeta Sporanaerobacter | [137] |
| BC | 1.0 g | AD system | Nitrate/Activated sludge | CV EIS CA Nyquist | Enhance microbial denitrification Promote nitrate reduction | Proteobacteria Bacteroidetes Firmicutes Pseudomona | [138] |
| CPBC | 2.0 g | AD system | Sludge/Food waste | Boehm titration 16S rRNA | Buffer acid Reduce lag time | Geobacter Syntroprobacter Methanosaeta | [59] |
| GAC | 0.2 g | AD system | Kitchen waste lipid-rapeseed oil | 16S rRNA | Improve CH4 yield Reduce acidification | Syntrophomonas Geobacter Methanosarcina | [139] |
| GAC | 5.0 g/L | AD system | Anaerobic sludge | 16S rRNA | Accelerate start-up Resist to organic load | Desulfuromonas Thermotogaceae Methanosarcina | [62] |
| GAC | 40.0 g/L | AD system | Anaerobic sludge | 16S rRNA | Improve CH4 yield Enhance COD removal | Synergistaceae Cloacibacillus | [140] |
| GAC | 75.0 g/L | UASB reactor | Incineration leachate | Metagenomic analysis | Resist to high organic load | Methanothrix Methanosarcina Geobacter | [141] |
| Graphite felt | SBR reactor | Anaerobic sludge | 16S rRNA | Accelerate VFAs degradation Promote microbial growth and CH4 production | Geobacter Methanosarcina | [15] | |
| Graphite powder | 7.0 wt% | An-IFFAS reactor | Anaerobic sludge | Four-probe measurement 16S rRNA | Promotes CH4 production | Geobacter Syntrophobacter Smithella Methanothrix | [9] |
| Acetylene Black | 1.0 g/L | AD system | Vinegar residue | 16S rRNA | Improve CH4 yield Promote acetate conversion | Syntrophomonadaceae Methanosarcinaceae | [18] |
| HC: hydrochar; BC: biochar; CPBC: citrus peel biochar; GAC: granular activated carbon. | |||||||
表选项
表 2. 产CH4体系中铁基CM的作用识别及富集微生物种类作用 Table 2. Roles of iron-based CM in CH4 production system with enriched functional microbes
| Type | Dosage | Inoculum | Substrate | Identification technique | Functions | Enriched microbes | References |
| S-nZVI | 0.3 g/L | AD system | Nitrobenzene | Tian et al. 16S rRNA CV | Enhance performance delivery Promote VFAs transformation Provide key enzyme activity | Caldisericum Methanosphaerula Methanomassiliicoccus | [107] |
| ZVI | 10.0 mmol/L | AD system | Anaerobic sludge | The phenol- sulfuric method Lowry-Folin method 16S rRNA | Improve CH4 yield Antitoxicity Be the role of substituted proteins | Syntrophomonas Tepidimicrobium Anaerosphaera | [96] |
| AC/ Magnetite | 5.0 g/L | UASB reactor | Coal gasification wastewater | Lowry-Folin method 16S rRNA | Accelerate syntrophic metabolism Improve CH4 production | Geobacter Methanothrix | [142] |
| Fe-Mn/BC | 1.5 g | AD system | Anaerobic sludge | 16S rRNA | Promote methanogens activity Alleviate ammonia inhibition | Methanosaeta Methanosarcina Methanobacterium | [55] |
| BC/S-nZVI | 1.0 g/L | UASB reactor | Nitrobenzene | 1, 10-phenanthroline 16S rRNA | Accelerate organic biodegradation Improve system stability | Bacteroides Longilinea Methanosarcina | [143] |
| Fe2O3/CC | AD system | Propionic acid | CER LSCV The chronoamperometry 16S rRNA | Improve CH4 yield Promote electron transfer | Levilinea Methanothrix Methanobacterium | [23] | |
| ZVI | 20.0 g/L | AD system | Anaerobic sludge | LDH The diphenylamine colorimetry | Promote electron transfer | Firmicutes Proteobacteria Bacteroidetes Oscillibacter Methanobacterium | [94] |
| S-nZVI | 10.0 g/L | AD system | Sludge/Food waste | ETS Lowry-Folin 16S rRNA | Improve CH4 yield Promote electron transfer | Euryarchaeota Chloroflexi Methanomicrobia | [96] |
| Fe/BC | 1.0 g/L | AD system | Nitrobenzene | EPR CV DPV | Promote nitrobenzene transformation Improve electrochemical activity | Geobacter sulfreducens | [144] |
| Magnetite/ BC | 30.0 mmol/L | AD system | Anaerobic sludge | CV 16S rRNA | Improve AD performance | Clostridiaceae Methanobacteriaceae Methanomicrobiaceae | [57] |
| Fe3O4/Coke | 1.0-3.0 mg/L | AD system | Coal pyrolysis wastewater | Metagenomic analysis | Enhance pollutant removal | Syntrophorhabdus Geobacter Alicycliphilus | [145] |
| S-nZVI: sulfidemodified nanoscale zero valent iron; ZVI: zero valent iron; AC: activated carbon; BC: biochar; CC: carbon cloth. | |||||||
表选项
Methanosaeta和Methanosarcina是最常见的两种利用乙酸盐产CH4的物种,也是具备细胞外电子交换能力的微生物。CM可以刺激这两类电活性产甲烷菌的富集以进行DIET。Methanosaeta利用乙酸盐产CH4,Methanosarcina既可以利用CO2产CH4,又可以将乙酸分解生成CH4,是一种多功能产甲烷古菌[135]。CM存在时,Methanosaeta和Methanosarcina都可以成为Geobacter的共营养体。Geobacter氧化有机物产生的电子传递到CM,再转移到Methanosaeta中用于消耗CO2产CH4,其中CM充当电子储存器的作用,这就是以DIET为基础的高效同营养产CH4反应体系[74]。在添加CM的嗜热(55 ℃)环境中Methanosaeta和Methanosarcina总比率是对照组的2倍[95]。Methanosaeta是对氨抑制最为敏感的厌氧菌,因此在氨抑制条件下维持Methanosaeta的生长优势是影响产CH4性能的关键,CM的添加就可起到上述作用,从而证明CM对DIET的促进作用[53]。除以上产甲烷古菌外,Methanolinea (甲烷绳菌属)、Methanobacterium (甲烷杆菌属)、Methanothrix (甲烷丝菌属)、Methanomassiliicoccus (甲烷马赛球菌)、Methanomethylovorans (甲烷嗜甲基菌属)的相对丰度在添加CM的厌氧体系中也有所增加(表 1、表 2)。其中Methanobacterium属于氢营养性产甲烷菌,可将CO2和H2转化为CH4,有研究表明Methanobacterium可以通过DIET直接接受电子产CH4[136]。Methanothrix可通过DIET与还原铁细菌建立电子连接产CH4[23]。DIET过程中接受电子的产甲烷古菌在CM强化体系内的种类及分布特征,还需深入研究。
4 结论和展望 DIET所涉及的微生物包括给电子细菌和产甲烷古菌,CM材料的作用是将给电子细菌氧化有机物释放的电子传递给产甲烷古菌,进而利用CO2产生CH4[44]。CM使参与DIET的物种间形成导电通路,并加速产甲烷菌的生长。生长速度较快的细菌可以更快地适应新环境,表现出较短的滞后时间,因此获得更高的甲烷产量和甲烷产率[146]。纳米级CM可穿透Methanosarcina barkeri的细胞膜和细胞质来加速细胞内电子传递,从而提高CH4产量[147]。CM强化产CH4过程有以下几个途径:(1) 为微生物提供适宜的生存环境和附着空间;(2) 吸附有毒物质减轻对微生物的抑制作用;(3) 促进中间产物VFAs的降解,避免微生物被毒害和系统酸化;(4) 加速产甲烷古菌的生长,促进给电子细菌和产甲烷古菌之间的电子传递;(5) 富集DIET功能微生物。但是,DIET作用在CM强化产CH4过程中的贡献率还有待进一步确认。
添加CM增强DIET促进产CH4作用是提升AD效率的有效途径。本文系统总结了最为常见的碳基与铁基CM对DIET性能的影响,CM的添加不仅可以提高产CH4速率,还可以缩短滞后时间,促进VFAs的降解,提高微生物活性,抵抗氨抑制作用和高有机负荷。同时,对参与DIET的微生物进行了系统梳理。目前对于DIET的研究还处于初期阶段,有一系列问题尚待解决,未来需重点突破以下几个方面:在机理层面,需探究DIET发生的直接证据。尽管CM可以通过DIET增强产CH4,但已报道的均为间接证据,至今尚未有佐证DIET发生的直接证据。在CM方面,需设计开发性能更优、更利于回收的导电材料,除碳基、铁基CM外,还可以关注其他类型的材料。在微生物层面,需进一步挖掘DIET功能微生物资源,开发并利用更多的DIET功能菌。在实际应用层面,除了产CH4,还可以利用其去除地质环境中的其他污染如硝酸盐、高价重金属等。
References
| [1] | Duan CH, Zhang CJ, Sun YH, Li M. Recent advances on the novel methanogens. Acta Microbiologica Sinica, 2019, 59(6): 981-995. (in Chinese) 段昌海, 张翠景, 孙艺华, 李猛. 新型产甲烷古菌研究进展. 微生物学报, 2019, 59(6): 981-995. |
| [2] | Neshat SA, Mohammadi M, Najafpour GD. Photosynthesis assisted anaerobic digestion of cattle manure leachate in a hybrid bioreactor: an integrated system for enhanced wastewater treatment and methane production. Chemical Engineering Journal, 2017, 330: 616-624. DOI:10.1016/j.cej.2017.08.001 |
| [3] | Lv W, Schanbacher FL, Yu ZT. Putting microbes to work in sequence: Recent advances in temperature-phased anaerobic digestion processes. Bioresource Technology, 2010, 101(24): 9409-9414. DOI:10.1016/j.biortech.2010.07.100 |
| [4] | Zhong W, Jiang YG, Shi L. Direct electron transfer between bacteria and archaea. Acta Microbiologica Sinica, 2020, 60(9): 2030-2038. (in Chinese) 钟雯, 蒋永光, 石良. 细菌与古菌之间的直接电子传递. 微生物学报, 2020, 60(9): 2030-2038. |
| [5] | Chen Y, Cheng JJ, Creamer KS. Inhibition of anaerobic digestion process: a review. Bioresource Technology, 2008, 99(10): 4044-4064. DOI:10.1016/j.biortech.2007.01.057 |
| [6] | Wu Y, Wang S, Liang DH, Li N. Conductive materials in anaerobic digestion: From mechanism to application. Bioresource Technology, 2020, 298: 122403. DOI:10.1016/j.biortech.2019.122403 |
| [7] | Morita M, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio, 2011, 2(4): 00159-11. |
| [8] | Jiang YF, Zhang BG, He C, Shi JX, Borthwick AGL, Huang XY. Synchronous microbial vanadium (Ⅴ) reduction and denitrification in groundwater using hydrogen as the sole electron donor. Water Research, 2018, 141: 289-296. DOI:10.1016/j.watres.2018.05.033 |
| [9] | Liu JF, Liu T, Chen S, Yu HT, Zhang YB, Quan X. Enhancing anaerobic digestion in anaerobic integrated floating fixed-film activated sludge (An-IFFAS) system using novel electron mediator suspended biofilm carriers. Water Research, 2020, 175: 115697. DOI:10.1016/j.watres.2020.115697 |
| [10] | Barua S, Dhar BR. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresource Technology, 2017, 244: 698-707. DOI:10.1016/j.biortech.2017.08.023 |
| [11] | Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature, 2005, 435(7045): 1098-1101. DOI:10.1038/nature03661 |
| [12] | Zhao ZQ, Zhang YB, Li Y, Dang Y, Zhu TT, Quan X. Potentially shifting from interspecies hydrogen transfer to direct interspecies electron transfer for syntrophic metabolism to resist acidic impact with conductive carbon cloth. Chemical Engineering Journal, 2017, 313: 10-18. DOI:10.1016/j.cej.2016.11.149 |
| [13] | Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science, 2010, 330(6009): 1413-1415. DOI:10.1126/science.1196526 |
| [14] | Lovley DR. Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy & Environmental Science, 2011, 4(12): 4896. |
| [15] | Zhang MY, Ma YQ, Ji DD, Li XY, Zhang JS, Zang LH. Synergetic promotion of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with graphite felt in anaerobic digestion. Bioresource Technology, 2019, 287: 121373. DOI:10.1016/j.biortech.2019.121373 |
| [16] | Liu FH, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. Promoting direct interspecies electron transfer with activated carbon. Energy & Environmental Science, 2012, 5(10): 8982. |
| [17] | Chen SS, Rotaru AE, Shrestha PM, Malvankar NS, Liu FH, Fan W, Nevin KP, Lovley DR. Promoting interspecies electron transfer with biochar. Scientific Reports, 2014, 4: 5019. |
| [18] | Guo WY, Li YQ, Zhao K, Xu Q, Jiang H, Zhou HJ. Performance and microbial community analysis of anaerobic digestion of vinegar residue with adding of acetylene black or hydrochar. Waste and Biomass Valorization, 2020, 11(7): 3315-3325. DOI:10.1007/s12649-019-00664-3 |
| [19] | Chen SS, Rotaru AE, Liu FH, Philips J, Woodard TL, Nevin KP, Lovley DR. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresource Technology, 2014, 173: 82-86. DOI:10.1016/j.biortech.2014.09.009 |
| [20] | Li LL, Tong ZH, Fang CY, Chu J, Yu HQ. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Research, 2015, 70: 1-8. DOI:10.1016/j.watres.2014.11.042 |
| [21] | Liu FH, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environmental Microbiology, 2015, 17(3): 648-655. DOI:10.1111/1462-2920.12485 |
| [22] | Huang WW, Yang F, Huang WL, Wang DX, Lei ZF, Zhang ZY. Weak magnetic field significantly enhances methane production from a digester supplemented with zero valent iron. Bioresource Technology, 2019, 282: 202-210. DOI:10.1016/j.biortech.2019.03.013 |
| [23] | Xu YG, Wang MW, Yu QL, Zhang YB. Enhancing methanogenesis from anaerobic digestion of propionate with addition of Fe oxides supported on conductive carbon cloth. Bioresource Technology, 2020, 302: 122796. DOI:10.1016/j.biortech.2020.122796 |
| [24] | Lin RC, Cheng J, Zhang JB, Zhou JH, Cen KF, Murphy JD. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresource Technology, 2017, 239: 345-352. DOI:10.1016/j.biortech.2017.05.017 |
| [25] | Ueki T, Nevin KP, Rotaru AE, Wang LY, Ward JE, Woodard TL, Lovley DR. GeobacterStrains expressing poorly conductive pili reveal constraints on direct interspecies electron transfer mechanisms. mBio, 2018, 9(4): 01273-18. |
| [26] | Yang YG, Li DB, Xu MY. Research progress in microbial extracellular long-distance electron transport networks. Acta Microbiologica Sinica, 2020, 60(9): 2072-2083. (in Chinese) 杨永刚, 李道波, 许玫英. 微生物胞外长距离电子传递网络研究进展. 微生物学报, 2020, 60(9): 2072-2083. |
| [27] | KumarA, Hsu LHH, Kavanagh P, Barrière F, Lens PNL, Lapinsonnière L, Lienhard VJH, Schr?der U, Jiang XC, Leech D. The ins and outs of microorganism-electrode electron transfer reactions. Nature Reviews Chemistry, 2017, 1(3): 1-13. |
| [28] | Costa NL, Clarke TA, Philipp LA, Gescher J, Louro RO, Paquete CM. Electron transfer process in microbial electrochemical technologies: The role of cell-surface exposed conductive proteins. Bioresource Technology, 2018, 255: 308-317. DOI:10.1016/j.biortech.2018.01.133 |
| [29] | Lovley DR, Holmes DE, Nevin KP. Dissimilatory Fe(Ⅲ) and Mn(Ⅳ) Reduction. Advances in Microbial Physiology, 2004, 49: 219-286. |
| [30] | Tikhonova TV, Popov VO. Structural and functional studies of multiheme cytochromes c involved in extracellular electron transport in bacterial dissimilatory metal reduction. Biochem istry: Moscow, 2014, 79(13): 1584-1601. DOI:10.1134/S0006297914130094 |
| [31] | Wang GY, Zhang BG, Li S, Yang M, Yin CC. Simultaneous microbial reduction of vanadium (Ⅴ) and chromium (Ⅵ) by Shewanella loihica PV-4. Bioresource Technology, 2017, 227: 353-358. DOI:10.1016/j.biortech.2016.12.070 |
| [32] | Nevin KP, Kim BC, Glaven RH, Johnson JP, Woodard TL, Methé BA, Didonato RJ, Covalla SF, Franks AE, Liu AN, Lovley DR. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS One, 2009, 4(5). |
| [33] | Lovley DR. Syntrophy Goes electric: direct interspecies electron transfer. Annual Review of Microbiology, 2017, 71: 643-664. DOI:10.1146/annurev-micro-030117-020420 |
| [34] | Liu X, Zhou SG. Electrical conductivity and application of microbial nanowires. Acta Microbiologica Sinica, 2020, 60(9): 2039-2061. (in Chinese) 刘星, 周顺桂. 微生物纳米导线的导电机制及功能. 微生物学报, 2020, 60(9): 2039-2061. |
| [35] | Malvankar NS, Lovley DR. Microbial nanowires for bioenergy applications. Current Opinion in Biotechnology, 2014, 27: 88-95. DOI:10.1016/j.copbio.2013.12.003 |
| [36] | Li Y, Tang YP, Xiong P, Zhang MQ, Deng QL, Liang DD, Zhao ZQ, Feng YJ, Zhang YB. High-efficiency methanogenesis via kitchen wastes served as ethanol source to establish direct interspecies electron transfer during anaerobic Co-digestion with waste activated sludge. Water Research, 2020, 176: 115763. DOI:10.1016/j.watres.2020.115763 |
| [37] | Yin QD, Gu MQ, Hermanowicz SW, Hu HY, Wu GX. Potential interactions between syntrophic bacteria and methanogens via type Ⅳ pili and quorum-sensing systems. Environment International, 2020, 138: 105650. DOI:10.1016/j.envint.2020.105650 |
| [38] | Wang FB, Gu YQ, O'Brien JP, Yi SM, Yalcin SE, Srikanth V, Shen C, Vu D, Ing NL, Hochbaum AI, Egelman EH, Malvankar NS. Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell, 2019, 177(2): 361-369. e10. DOI:10.1016/j.cell.2019.03.029 |
| [39] | Baek G, Kim J, Kim J, Lee C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies, 2018, 11(1): 107. DOI:10.3390/en11010107 |
| [40] | Park JH, Kang HJ, Park KH, Park HD. Direct interspecies electron transfer via conductive materials: a perspective for anaerobic digestion applications. Bioresource Technology, 2018, 254: 300-311. DOI:10.1016/j.biortech.2018.01.095 |
| [41] | Zhang BG, Li YN, Fei YM, Cheng YT. Novel pathway for vanadium (Ⅴ) bio-detoxification by gram-positive Lactococcus raffinolactis. Environmental Science & Technology, 2021, 55(3): 2121-2131. |
| [42] | He C, Zhang BG, Lu JP, Qiu R. A newly discovered function of nitrate reductase in chemoautotrophic vanadate transformation by natural mackinawite in aquifer. Water Research, 2021, 189: 116664. DOI:10.1016/j.watres.2020.116664 |
| [43] | Zhang BG, Qiu R, Lu L, Chen X, He C, Lu JP, Ren ZJ. Autotrophic Vanadium(Ⅴ) Bioreduction in Groundwater by Elemental Sulfur and Zerovalent Iron. Environmental Science & Technology, 2018, 52(13): 7434-7442. |
| [44] | Gahlot P, Ahmed B, Tiwari SB, Aryal N, Khursheed A, Kazmi AA, Tyagi VK. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environmental Technology & Innovation, 2020, 20: 101056. |
| [45] | Rotaru AE, Shrestha PM, Liu FH, Markovaite B, Chen SS, Nevin KP, Lovley DR. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Applied and Environmental Microbiology, 2014, 80(15): 4599-4605. DOI:10.1128/AEM.00895-14 |
| [46] | Jing YH, Wan JJ, Angelidaki I, Zhang SC, Luo G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Research, 2017, 108: 212-221. DOI:10.1016/j.watres.2016.10.077 |
| [47] | Kato S, Nakamura R, Kai F, Watanabe K, Hashimoto K. Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environmental Microbiology, 2010, 12(12): 3114-3123. DOI:10.1111/j.1462-2920.2010.02284.x |
| [48] | Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for Biofilms. Applied and Environmental Microbiology, 2005, 71(8): 4414-4426. DOI:10.1128/AEM.71.8.4414-4426.2005 |
| [49] | Kostarelos K, Novoselov KS. Exploring the interface of graphene and biology. Science, 2014, 344(6181): 261-263. DOI:10.1126/science.1246736 |
| [50] | Lu PL, Wang XW, Tang YS, Ding A, Yang H, Guo JL, Cui Y, Ling CX. Granular activated carbon assisted nitrate-dependent anaerobic methane oxidation-membrane bioreactor: Strengthening effect and mechanisms. Environment International, 2020, 138: 105675. DOI:10.1016/j.envint.2020.105675 |
| [51] | Sohi SP, Krull E, Lopez-Capel E, Bol R. A review of biochar and its use and function in soil. Advances in Agronomy, 2010, 105: 47-82. |
| [52] | Hu QY, Zhu YL, Hu BW, Lu SH, Sheng GD. Mechanistic insights into sequestration of U(Ⅵ) toward magnetic biochar: Batch, XPS and EXAFS techniques. Journal of Environmental Scie nces, 2018, 70: 217-225. DOI:10.1016/j.jes.2018.01.013 |
| [53] | Ren S, Usman M, Tsang DCW, O-Thong S, Angelidaki I, Zhu XD, Zhang SC, Luo G. Hydrochar-facilitated anaerobic digestion: evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environmental Science & Technology, 2020, 54(9): 5755-5766. |
| [54] | Zhang DJ, Shen JY, Shi HF, Su GY, Jiang XB, Li JS, Liu XD, Mu Y, Wang LJ. Substantially enhanced anaerobic reduction of nitrobenzene by biochar stabilized sulfide-modified nanoscale zero-valent iron: Process and mechanisms. Environment International, 2019, 131: 105020. DOI:10.1016/j.envint.2019.105020 |
| [55] | Zhang M, Wang YC. Effects of Fe-Mn-modified biochar addition on anaerobic digestion of sewage sludge: Biomethane production heavy metal speciation and performance stability. Bioresource Technology, 2020, 313: 123695. DOI:10.1016/j.biortech.2020.123695 |
| [56] | Zhuang HF, Zhu H, Zhang J, Shan SD, Fang CR, Tang HJ, Xie QN. Enhanced 2, 4, 6-trichlorophenol anaerobic degradation by Fe3O4 supported on water hyacinth biochar for triggering direct interspecies electron transfer and its use in coal gasification wastewater treatment. Bioresource Technology, 2020, 296: 122306. DOI:10.1016/j.biortech.2019.122306 |
| [57] | Wang MW, Zhao ZQ, Zhang YB. Magnetite-contained biochar derived from fenton sludge modulated electron transfer of microorganisms in anaerobic digestion. Journal of Hazardous Materials, 2021, 403: 123972. DOI:10.1016/j.jhazmat.2020.123972 |
| [58] | Yan S, Cheng KY, Ginige MP, Zheng GY, Zhou LX, Kaksonen AH. Optimization of nitrate and selenate reduction in an ethanol-fed fluidized bed reactor via redox potential feedback control. Journal of Hazardous Materials, 2021, 402: 123770. DOI:10.1016/j.jhazmat.2020.123770 |
| [59] | Jiang Q, Chen YD, Yu SK, Zhu RL, Zhong C, Zou HJ, Gu L, He Q. Effects of Citrus peel biochar on anaerobic co-digestion of food waste and sewage sludge and its direct interspecific electron transfer pathway study. Chemical Engineering Journal, 2020, 398: 125643. DOI:10.1016/j.cej.2020.125643 |
| [60] | Dang Y, Holmes DE, Zhao ZQ, Woodard TL, Zhang YB, Sun DZ, Wang LY, Nevin KP, Lovley DR. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresource Technology, 2016, 220: 516-522. DOI:10.1016/j.biortech.2016.08.114 |
| [61] | Lee JY, Lee SH, Park HD. Enrichment of specific electro-active microorganisms and enhancement of methane production by adding granular activated carbon in anaerobic reactors. Bioresource Technology, 2016, 205: 205-212. DOI:10.1016/j.biortech.2016.01.054 |
| [62] | Xu SY, He CQ, Luo LW, Lü F, He PJ, Cui LF. Comparing activated carbon of different particle sizes on enhancing methane generation in upflow anaerobic digester. Bioresource Technology, 2015, 196: 606-612. DOI:10.1016/j.biortech.2015.08.018 |
| [63] | Yan WW, Shen N, Xiao YY, Chen Y, Sun FQ, Kumar Tyagi V, Zhou Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresource Technology, 2017, 239: 336-344. DOI:10.1016/j.biortech.2017.05.046 |
| [64] | Romero RM, Valenzuela EI, Cervantes FJ, Garcia-Reyes RB, Serrano D, Alvarez LH. Improved methane production from anaerobic digestion of liquid and raw fractions of swine manure effluent using activated carbon. Journal of Water Process Engineering, 2020, 38: 101576. DOI:10.1016/j.jwpe.2020.101576 |
| [65] | Tan LC, Lin RC, Murphy JD, Lens PNL. Granular activated carbon supplementation enhances anaerobic digestion of lipid-rich wastewaters. Renewable Energy, 2021, 171: 958-970. DOI:10.1016/j.renene.2021.02.087 |
| [66] | Yang YF, Zhang YB, Li ZY, Zhao ZQ, Quan X, Zhao ZS. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. Journal of Cleaner Production, 2017, 149: 1101-1108. DOI:10.1016/j.jclepro.2017.02.156 |
| [67] | Peng H, Zhang YB, Tan DM, Zhao ZQ, Zhao HM, Quan X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresource Technology, 2018, 249: 666-672. DOI:10.1016/j.biortech.2017.10.047 |
| [68] | Dang Y, Sun DZ, Woodard TL, Wang LY, Nevin KP, Holmes DE. Stimulation of the anaerobic digestion of the dry organic fraction of municipal solid waste (OFMSW) with carbon-based conductive materials. Bioresource Technology, 2017, 238: 30-38. DOI:10.1016/j.biortech.2017.04.021 |
| [69] | Jiang Q, Liu H, Zhang Y, Cui MH, Fu B, Liu HB. Insight into sludge anaerobic digestion with granular activated carbon addition: Methanogenic acceleration and methane reduction relief. Bioresource Technology, 2021, 319: 124131. DOI:10.1016/j.biortech.2020.124131 |
| [70] | Johnravindar D, Liang BB, Fu RZ, Luo G, Meruvu H, Yang SM, Yuan B, Fei Q. Supplementing granular activated carbon for enhanced methane production in anaerobic co-digestion of post-consumer substrates. Biomass and Bioenergy, 2020, 136: 105543. DOI:10.1016/j.biombioe.2020.105543 |
| [71] | He X, Guo ZY, Lu J, Zhang R. Carbon-based conductive materials accelerated methane production in anaerobic digestion of waste fat, oil and grease. Bioresource Technology, 2021, 329: 124871. DOI:10.1016/j.biortech.2021.124871 |
| [72] | He CH, Liu TX, Ou H, Yuan SJ, Hu ZH, Wang W. Coupling granular activated carbon and exogenous hydrogen to enhance anaerobic digestion of phenol via predominant syntrophic acetate oxidation and hydrogenotrophic methanogenesis pathway. Bioresource Technology, 2021, 323: 124576. DOI:10.1016/j.biortech.2020.124576 |
| [73] | Zhao ZQ, Zhang YB, Woodard TL, Nevin KP, Lovley DR. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresource Technology, 2015, 191: 140-145. DOI:10.1016/j.biortech.2015.05.007 |
| [74] | Wang GJ, Gao X, Li Q, Zhao HX, Liu YZ, Wang XC, Chen R. Redox-based electron exchange capacity of biowaste-derived biochar accelerates syntrophic phenol oxidation for methanogenesis via direct interspecies electron transfer. Journal of Hazardous Materials, 2020, 390: 121726. DOI:10.1016/j.jhazmat.2019.121726 |
| [75] | Jiang HM, Choi YK, Kan E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresource Technology, 2018, 250: 927-931. DOI:10.1016/j.biortech.2017.11.074 |
| [76] | Ambaye TG, Rene ER, Dupont C, Wongrod S, van Hullebusch ED. Anaerobic digestion of fruit waste mixed with sewage sludge digestate biochar: influence on biomethane production. Frontiers in Energy Research, 2020, 8: 31. DOI:10.3389/fenrg.2020.00031 |
| [77] | Lü F, Luo CH, Shao LM, He PJ. Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Research, 2016, 90: 34-43. DOI:10.1016/j.watres.2015.12.029 |
| [78] | Cruz Viggi C, Simonetti S, Palma E, Pagliaccia P, Braguglia C, Fazi S, Baronti S, Navarra MA, Pettiti I, Koch C, Harnisch F, Aulenta F. Enhancing methane production from food waste fermentate using biochar: the added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnology for B iofuels, 2017, 10(1): 1-13. DOI:10.1186/s13068-017-0994-7 |
| [79] | Li Q, Xu MJ, Wang GJ, Chen R, Qiao W, Wang XC. Biochar assisted thermophilic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresource Technology, 2018, 249: 1009-1016. DOI:10.1016/j.biortech.2017.11.002 |
| [80] | Sugiarto Y, Sunyoto NMS, Zhu MM, Jones I, Zhang DK. Effect of biochar addition on microbial community and methane production during anaerobic digestion of food wastes: The role of minerals in biochar. Bioresource Technology, 2021, 323: 124585. DOI:10.1016/j.biortech.2020.124585 |
| [81] | Cui YX, Mao FJ, Zhang JX, He YL, Tong YW, Peng YH. Biochar enhanced high-solid mesophilic anaerobic digestion of food waste: Cell viability and methanogenic pathways. Chemospher e, 2021, 272: 129863. DOI:10.1016/j.chemosphere.2021.129863 |
| [82] | Wang ZQ, Yun SN, Xu HF, Wang C, Zhang YL, Chen JG, Jia B. Mesophilic anaerobic co-digestion of acorn slag waste with dairy manure in a batch digester: Focusing on mixing ratios and bio-based carbon accelerants. Bioresource Technology, 2019, 286: 121394. DOI:10.1016/j.biortech.2019.121394 |
| [83] | Tian T, Qiao S, Li X, Zhang MJ, Zhou JT. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresource Technology, 2017, 224: 41-47. DOI:10.1016/j.biortech.2016.10.058 |
| [84] | Murat?obano?lu H, G?k?ek ?B, Mert RA, Zan R, Demirel S. Simultaneous synergistic effects of graphite addition and co-digestion of food waste and cow manure: Biogas production and microbial community. Bioresource Technology, 2020, 309: 123365. DOI:10.1016/j.biortech.2020.123365 |
| [85] | Xiao LL, Zheng SL, Lichtfouse E, Luo M, Tan Y, Liu FH. Carbon nanotubes accelerate acetoclastic methanogenesis: From pure cultures to anaerobic soils. Soil Biology and Biochemistry, 2020, 150: 107938. DOI:10.1016/j.soilbio.2020.107938 |
| [86] | Ambuchi JJ, Zhang ZH, Shan LL, Liang DD, Zhang P, Feng YJ. Response of anaerobic granular sludge to iron oxide nanoparticles and multi-wall carbon nanotubes during beet sugar industrial wastewater treatment. Water Research, 2017, 117: 87-94. DOI:10.1016/j.watres.2017.03.050 |
| [87] | Yan WW, Lu D, Liu JB, Zhou Y. The interactive effects of ammonia and carbon nanotube on anaerobic digestion. Chemical Engineering Journal, 2019, 372: 332-340. DOI:10.1016/j.cej.2019.04.163 |
| [88] | Hao Y, Wang YY, Ma CX, White JC, Zhao ZQ, Duan C, Zhang YL, Adeel M, Rui YK, Li GX, Xing BS. Carbon nanomaterials induce residue degradation and increase methane production from livestock manure in an anaerobic digestion system. Journal of Cleaner Production, 2019, 240: 118257. DOI:10.1016/j.jclepro.2019.118257 |
| [89] | Wang FB, Wang J, Li ZL, Zan SJ, Du MM. Promoting anaerobic digestion by algae-based hydrochars in a continuous reactor. Bioresource Technology, 2020, 318: 124201. DOI:10.1016/j.biortech.2020.124201 |
| [90] | Pagés-Díaz J, Huili?ir C. Valorization of the liquid fraction of co-hydrothermal carbonization of mixed biomass by anaerobic digestion: Effect of the substrate to inoculum ratio and hydrochar addition. Bioresource Technology, 2020, 317: 123989. DOI:10.1016/j.biortech.2020.123989 |
| [91] | Usman M, Shi ZJ, Ren S, Ngo HH, Luo G, Zhang SC. Hydrochar promoted anaerobic digestion of hydrothermal liquefaction wastewater: Focusing on the organic degradation and microbial community. Chemical Engineering Journal, 2020, 399: 125766. DOI:10.1016/j.cej.2020.125766 |
| [92] | Igarashi K, Miyako E, Kato S. Direct interspecies electron transfer mediated by graphene oxide-based materials. Frontiers in Microbiology, 2019, 10: 3068. |
| [93] | Shen N, Liang Z, Chen Y, Song HL, Wan JF. Enhancement of syntrophic acetate oxidation pathway via single walled carbon nanotubes addition under high acetate concentration and thermophilic condition. Bioresource Technology, 2020, 306: 123182. DOI:10.1016/j.biortech.2020.123182 |
| [94] | Wang Y, We iW, Wu SL, Ni BJ. Zero Valent Iron Effectively Enhances Medium-Chain Fatty Acids Production from Waste Activated Sludge through Improving Sludge Biodegradability and Electron Transfer Efficiency. Environmental Science Technology, 2020, 27: 20036. |
| [95] | Yan WW, Mukherjee M, Zhou Y. Direct interspecies electron transfer (DIET) can be suppressed under ammonia-stressed condition-Reevaluate the role of conductive materials. Water Research, 2020, 183: 116094. DOI:10.1016/j.watres.2020.116094 |
| [96] | Chen SJ, Tao Z, Yao FB, Wu B, He L, Hou KJ, Pi ZJ, Fu J, Yin HY, Huang Q, Liu YJ, Wang DB, Li XM, Yang Q. Enhanced anaerobic co-digestion of waste activated sludge and food waste by sulfidated microscale zerovalent iron: Insights in direct interspecies electron transfer mechanism. Bioresource Technology, 2020, 316: 123901. DOI:10.1016/j.biortech.2020.123901 |
| [97] | Zhao ZS, Zhang YB, Li Y, Quan X, Zhao ZQ. Comparing the mechanisms of ZVI and Fe3O4 for promoting waste-activated sludge digestion. Water Research, 2018, 144: 126-133. DOI:10.1016/j.watres.2018.07.028 |
| [98] | Jin Z, Zhao ZQ, Zhang YB. Potential of direct interspecies electron transfer in synergetic enhancement of methanogenesis and sulfate removal in an up-flow anaerobic sludge blanket reactor with magnetite. Science of the Total Environment, 2019, 677: 299-306. DOI:10.1016/j.scitotenv.2019.04.372 |
| [99] | Jiang ZM, Lv L, Zhang WM, Du Q, Pan BC, Yang L, Zhang QX. Nitrate reduction using nanosized zero-valent iron supported by polystyrene resins: Role of surface functional groups. Water Research, 2011, 45(6): 2191-2198. DOI:10.1016/j.watres.2011.01.005 |
| [100] | Shi JX, Zhang BG, Qiu R, Lai CY, Jiang YF, He C, Guo JH. Microbial chromate reduction coupled to anaerobic oxidation of elemental sulfur or zerovalent iron. Environmental Science & Technology, 2019, 53(6): 3198-3207. |
| [101] | Liu YW, Zhang YB, Quan X, Li Y, Zhao ZQ, Meng XS, Chen S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chemical Engineering Journal, 2012, 192: 179-185. DOI:10.1016/j.cej.2012.03.044 |
| [102] | Fan DM, Lan Y, Tratnyek PG, Johnson RL, Filip J, O'Carroll DM, Nunez Garcia A, Agrawal A. Sulfidation of iron-based materials: a review of processes and implications for water treatment and remediation. Environmental Science Technology, 2017, 51(22): 13070-13085. DOI:10.1021/acs.est.7b04177 |
| [103] | Li D, Mao Z, Zhong Y, Huang WL, Wu YD, Peng PA. Reductive transformation of tetrabromobisphenol A by sulfidated nano zerovalent iron. Water Research, 2016, 103: 1-9. DOI:10.1016/j.watres.2016.07.003 |
| [104] | Cheng YJ, Dong HR, Lu Y, Hou KJ, Wang YY, Ning Q, Li L, Wang B, Zhang LH, Zeng GM. Toxicity of sulfide-modified nanoscale zero-valent iron to Escherichia coli in aqueous solutions. Chemosphere, 2019, 220: 523-530. DOI:10.1016/j.chemosphere.2018.12.159 |
| [105] | Cruz Viggi C, Rossetti S, Fazi S, Paiano P, Majone M, Aulenta F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environmental Science & Technology, 2014, 48(13): 7536-7543. |
| [106] | Kato S, Hashimoto K, Watanabe K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environmental Microbiology, 2012, 14(7): 1646-1654. DOI:10.1111/j.1462-2920.2011.02611.x |
| [107] | Zhang DJ, Li Y, Sun AW, Tong SQ, Jiang XB, Mu Y, Li JS, Han WQ, Sun XY, Wang LJ, Shen JY. Optimization ofS/Fe ratio for enhanced nitrobenzene biological removal in anaerobicSystem amended withSulfide-modified nanoscale zerovalent iron. Chemosphere, 2020, 247: 125832. DOI:10.1016/j.chemosphere.2020.125832 |
| [108] | Yin QD, Miao J, Li B, Wu GX. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. International Biodeterioration & Biodegradation, 2017, 119: 104-110. |
| [109] | Yin QD, Yang S, Wang ZZ, Xing LZ, Wu GX. Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide. Chemical Engineering Journal, 2018, 333: 216-225. DOI:10.1016/j.cej.2017.09.160 |
| [110] | Baek G, Jung H, Kim J, Lee C. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent-Magnetic separation and recycling of magnetite. Bioresource Technology, 2017, 241: 830-840. DOI:10.1016/j.biortech.2017.06.018 |
| [111] | Lei YQ, Wei LX, Liu TY, Xiao YY, Dang Y, Sun DZ, Holmes DE. Magnetite enhances anaerobic digestion and methanogenesis of fresh leachate from a municipal solid waste incineration plant. Chemical Engineering Journal, 2018, 348: 992-999. DOI:10.1016/j.cej.2018.05.060 |
| [112] | Wang DX, Han YX, Han HJ, Li K, Xu CY, Zhuang HF. New insights into enhanced anaerobic degradation of Fischer-Tropsch wastewater with the assistance of magnetite. Bioresource Technology, 2018, 257: 147-156. DOI:10.1016/j.biortech.2018.02.084 |
| [113] | Sun MC, Zhang ZH, Liu GH, Lv M, Feng YJ. Enhancing methane production of synthetic brewery water with granular activated carbon modified with nanoscale zero-valent iron (NZVI) in anaerobic system. Science of the Total Environment, 2021, 760: 143933. DOI:10.1016/j.scitotenv.2020.143933 |
| [114] | Wang RM, Li CX, Lv N, Pan XF, Cai GJ, Ning J, Zhu GF. Deeper insights into effect of activated carbon and nano-zero-valent iron addition on acidogenesis and whole anaerobic digestion. Bioresource Technology, 2021, 324: 124671. DOI:10.1016/j.biortech.2021.124671 |
| [115] | Xu H, Liu YB, Yang B, Jia LJ, Li X, Li F, Song XS, Cao X, Sand W. Inhibitory effect of released phosphate on the ability of nano zero valent iron to boost anaerobic digestion of waste-activated sludge and the remediation method. Chemical Engineering Journal, 2021, 405: 126506. DOI:10.1016/j.cej.2020.126506 |
| [116] | Pan XF, Lv N, Li CX, Ning J, Wang T, Wang RM, Zhou MD, Zhu GF. Impact of nano zero valent iron on tetracycline degradation and microbial community succession during anaerobic digestion. Chemical Engineering Journal, 2019, 359: 662-671. DOI:10.1016/j.cej.2018.11.135 |
| [117] | Feng YH, Zhang YB, Quan X, Chen S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Research, 2014, 52: 242-250. DOI:10.1016/j.watres.2013.10.072 |
| [118] | Charalambous P, Vyrides I. In situ biogas upgrading and enhancement of anaerobic digestion of cheese whey by addition of scrap or powder zero-valent iron (ZVI). Journal of Environmental Management, 2021, 280: 111651. DOI:10.1016/j.jenvman.2020.111651 |
| [119] | Yang Y, Yang F, Huang WW, Huang WL, Li F, Lei ZF, Zhang ZY. Enhanced anaerobic digestion of ammonia-rich swine manure by zero-valent iron: With special focus on the enhancement effect on hydrogenotrophic methanogenesis activity. Bioresource Technology, 2018, 270: 172-179. DOI:10.1016/j.biortech.2018.09.008 |
| [120] | Zhang YB, Feng YH, Quan X. Zero-valent iron enhanced methanogenic activity in anaerobic digestion of waste activated sludge after heat and alkali pretreatment. Waste Management, 2015, 38: 297-302. DOI:10.1016/j.wasman.2015.01.036 |
| [121] | Ambuchi JJ, Zhang ZH, Dong Y, Huang LL, Feng YJ. Hematite and multi-walled carbon nanotubes stimulate a faster syntrophic pathway during methanogenic beet sugar industrial wastewater degradation. Applied Microbiology and Biotechnology, 2018, 102(16): 7147-7158. DOI:10.1007/s00253-018-9100-8 |
| [122] | Zhuang L, Tang J, Wang YQ, Hu M, Zhou SG. Conductive iron oxide minerals accelerate syntrophic cooperation in methanogenic benzoate degradation. Journal of Hazardous Materials, 2015, 293: 37-45. DOI:10.1016/j.jhazmat.2015.03.039 |
| [123] | Jiang SH, Park S, Yoon Y, Lee JH, Wu WM, Phuoc Dan N, Sadowsky MJ, Hur HG. Methanogenesis facilitated by geobiochemical iron Cycle in a novel Syntrophic methanogenic microbial community. Environmental Science & Technology, 2013, 47(17): 10078-10084. |
| [124] | Cheng X, Chen B, Cui YX, Sun DZ, Wang XZ. Iron(Ⅲ) reduction-induced phosphate precipitation during anaerobic digestion of waste activated sludge. Separation and Purification Technology, 2015, 143(25): 6-11. |
| [125] | Tang YP, Li Y, Zhang MQ, Xiong P, Liu LF, Bao YM, Zhao ZQ. Link between characteristics of Fe(Ⅲ) oxides and critical role in enhancing anaerobic methanogenic degradation of complex organic compounds. Environmental Research, 2021, 194: 110498. DOI:10.1016/j.envres.2020.110498 |
| [126] | Lu TD, Zhang JY, Wei YS, Shen PH. Effects of ferric oxide on the microbial community and functioning during anaerobic digestion of swine manure. Bioresource Technology, 2019, 287: 121393. DOI:10.1016/j.biortech.2019.121393 |
| [127] | Ye J, Hu AD, Cheng XY, Lin WF, Liu X, Zhou SG, He Z. Response of enhanced sludge methanogenesis by red mud to temperature: Spectroscopic and electrochemical elucidation of endogenous redox mediators. Water Research, 2018, 143: 240-249. DOI:10.1016/j.watres.2018.06.061 |
| [128] | Ye J, Hu AD, Ren GP, Chen M, Tang JH, Zhang PY, Zhou SG, He Z. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud. Water Research, 2018, 134: 54-62. DOI:10.1016/j.watres.2018.01.062 |
| [129] | Ye J, Hu AD, Ren GP, Zhou T, Zhang GM, Zhou SG. Red mud enhances methanogenesis with the simultaneous improvement of hydrolysis-acidification and electrical conductivity. Bioresource Technology, 2018, 247: 131-137. DOI:10.1016/j.biortech.2017.08.063 |
| [130] | Zhang LS, Keller J, Yuan ZG. Inhibition of sulfate-reducing and methanogenic activities of anaerobic sewer biofilms by ferric iron dosing. Water Research, 2009, 43(17): 4123-4132. DOI:10.1016/j.watres.2009.06.013 |
| [131] | Zhao ZS, Li Y, Yu QL, Zhang YB. Ferroferric oxide triggered possible direct interspecies electron transfer between Syntrophomonas and Methanosaeta to enhance waste activated sludge anaerobic digestion. Bioresource Technology, 2018, 250: 79-85. DOI:10.1016/j.biortech.2017.11.003 |
| [132] | Saha S, Basak B, Hwang JH, Salama ES, Chatterjee PK, Jeon BH. Microbial symbiosis: a network towards biomethanation. Trends in Microbiology, 2020, 28(12): 968-984. DOI:10.1016/j.tim.2020.03.012 |
| [133] | Qi QX, Sun C, Zhang JX, He YL, Wash Tong Y. Internal enhancement mechanism of biochar with graphene structure in anaerobic digestion: The bioavailability of trace elements and potential direct interspecies electron transfer. Chemical Engineering Journal, 2021, 406: 126833. DOI:10.1016/j.cej.2020.126833 |
| [134] | Mostafa A, Im S, Song YC, Ahn Y, Kim DH. Enhanced anaerobic digestion by stimulating DIET reaction. Processes, 2020, 8(4): 424. DOI:10.3390/pr8040424 |
| [135] | Guo JH, Peng YZ, Ni BJ, Han XY, Fan L, Yuan ZG. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microbial Cell Factories, 2015, 14(1): 1-11. DOI:10.1186/s12934-014-0183-3 |
| [136] | Lin RC, Cheng J, Ding LK, Murphy JD. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chemical Engineering Journal, 2018, 350: 681-691. DOI:10.1016/j.cej.2018.05.173 |
| [137] | Li Y, Liu MS, Che XR, Li C, Liang DD, Zhou H, Liu LF, Zhao ZQ, Zhang YB. Biochar stimulates growth of novel species capable of direct interspecies electron transfer in anaerobic digestion via ethanol-type fermentation. Environmental Research, 2020, 189: 109983. DOI:10.1016/j.envres.2020.109983 |
| [138] | Sathishkumar K, Li Y, Sanganyado E. Electrochemical behavior of biochar and its effects on microbial nitrate reduction: Role of extracellular polymeric substances in extracellular electron transfer. Chemical Engineering Journal, 2020, 395: 125077. DOI:10.1016/j.cej.2020.125077 |
| [139] | Zhang J, Zhang RT, Wang HY, Yang K. Direct interspecies electron transfer stimulated by granular activated carbon enhances anaerobic methanation efficiency from typical kitchen waste lipid-rapeseed oil. Science of the Total Environment, 2020, 704: 135282. DOI:10.1016/j.scitotenv.2019.135282 |
| [140] | Zhao ZQ, Li Y, Quan X, Zhang YB. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Research, 2017, 115: 266-277. DOI:10.1016/j.watres.2017.02.067 |
| [141] | Lei YQ, Sun DZ, Dang Y, Feng XL, Huo D, Liu CQ, Zheng K, Holmes DE. Metagenomic analysis reveals that activated carbon aids anaerobic digestion of raw incineration leachate by promoting direct interspecies electron transfer. Water Research, 2019, 161(15): 570-580. |
| [142] | Zhuang HF, Xie QN, Shan SD, Fang CR, Ping LF, Zhang CG, Wang ZR. Performance, mechanism and stability of nitrogen-doped sewage sludge based activated carbon supported magnetite in anaerobic degradation of coal gasification wastewater. Science of the Total Environment, 2020, 737: 140285. DOI:10.1016/j.scitotenv.2020.140285 |
| [143] | Zhang DJ, Li Y, Tong SQ, Jiang XB, Wang LJ, Sun XY, Li JS, Liu XD, Shen JY. Biochar supported sulfide-modified nanoscale zero-valent iron for the reduction of nitrobenzene. RSC Advances, 2018, 8(39): 22161-22168. DOI:10.1039/C8RA04314K |
| [144] | Lu Y, Xie QQ, Tang L, Yu JF, Wang JJ, Yang ZH, Fan CZ, Zhang SJ. The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge. Journal of Hazardous Materials, 2021, 403: 123682. DOI:10.1016/j.jhazmat.2020.123682 |
| [145] | Zheng MQ, Han HJ, Shi JX, Zhang ZW, Ma WC, Xu CY. Metagenomic analysis of aromatic ring-cleavage mechanism in nano-Fe3O4@activated coke enhanced bio-system for coal pyrolysis wastewater treatment. Journal of Hazardous Materials, 2021, 414: 125387. DOI:10.1016/j.jhazmat.2021.125387 |
| [146] | Fu L, Zhou T, Wang JY, You LX, Lu YH, Yu LP, Zhou SG. Nano Fe3O4 as solid electron shuttles to accelerate acetotrophic methanogenesis by Methanosarcina barkeri. Frontiers in Microbiology, 2019, 10: 388. DOI:10.3389/fmicb.2019.00388 |
| [147] | Mohapatra BR, Dinardo O, Gould WD, Koren DW. Biochemical and genomic facets on the dissimilatory reduction of radionuclides by microorganisms-A review. Minerals Engineering, 2010, 23(8): 591-599. DOI:10.1016/j.mineng.2010.03.004 |
