唐玉景, 龙旭伟


南京理工大学环境与生物工程学院, 江苏南京 210094
收稿日期:2020-06-18;修回日期:2020-10-07;网络出版日期:2021-01-19
基金项目:国家自然科学基金(51778293)
*通信作者:龙旭伟, E-mail: xuweilong@njust.edu.cn.
摘要:槐糖脂是一类由酵母菌代谢生产的糖脂型生物表面活性剂,具有优越的表/界面活性和稳定性,且无毒、无刺激、易被生物降解,在众多领域具有良好的应用前景,被认为是最具潜力替代化学表面活性剂的生物表面活性剂之一。历经50余年的研究,槐糖脂的发酵生产工艺日趋成熟,但产品的分离纯化仍是难点。本文系统地综述了槐糖脂的结构、生物合成途径,重点关注近年来槐糖脂的发酵生产研究现状,以及分离纯化研究现状。并对槐糖脂的发酵生产和分离研究进行了展望,旨在促进槐糖脂的规模化生产与市场化发展。
关键词:槐糖脂生物合成发酵生产分离纯化
Biosynthesis and downstream processing of biosurfactant sophorolipids
Yujing Tang, Xuwei Long


School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu Province, China
Received: 18 June 2020; Revised: 7 October 2020; Published online: 19 January 2021
*Corresponding author: Xuwei Long, E-mail: xuweilong@njust.edu.cn.
Foundation item: Supported by the National Natural Science Foundation ofChina (51778293)
Abstract: Sophorolipids are glycolipids biosurfactants largely produced by various yeasts. They have excellent surface/interfacial activities and chemical stability even at extreme pH, temperature and salinity. More important, sophorolipids have many advantages over the chemical surfactants including non-toxic, non-irritating and easy-to-biodegrade, with many potential applications in many fields. Through the efforts made in the past five decades, the fermentation level of sophorolipids has been highly improved, but the downstream processing that counts for 60% to 80% of the total production cost, is still a challenge. This review highlights the properties and structures of sophorolipids, the biosynthesis pathway of sophorolipids, and the progress in fermentation and separation of sophorolipids in recent years. In addition, the prospects doe sophorolipids fermentation and separation towards their industrialization and commercialization are also addressed.
Keywords: sophorolipidsbiosynthesisfermentationseparation and purification
槐糖脂(Sophorolipids,SLs)是一类由酵母菌代谢产生的糖脂类生物表面活性剂,具有低毒性、可生物降解、对环境友好等特性,是目前研究最多,也最具有市场前景的生物表面活性剂之一[1]。槐糖脂是由Gorin等于1961年在酵母菌Torulopsis magnoliae培养液中首次发现[2],该酵母菌在1968年被重新鉴定为Torulopsis apicola,也称Candida apicola[3]。后又陆续发现Starmerella bombicola等真菌也可分泌槐糖脂[1, 4]。迄今为止,欧洲的Evonik、DSM和Croda公司已经实现了槐糖脂的工业化生产[5];日本的Saraya公司和比利时的Ecover公司也已经将槐糖脂应用到洗涤剂产品中,而法国的Soliance公司(已并入瑞士的Givaudan公司)和韩国的MG Intobio公司在化妆品产品中添加了槐糖脂[5]。可见,槐糖脂的市场前景十分广阔。
天然酵母菌生产的槐糖脂有20种主要结构以及100种次要结构[1]。槐糖脂结构(图 1)的复杂性主要取决于槐糖脂酸型和内酯型结构的不同、槐糖分子上6′和/或6″位置乙酰化程度的不同、槐糖所连接脂肪酸种类的不同以及脂肪酸链与糖原连接位点的差异(ω或ω-1位点连接等)。槐糖脂具有卓越的表/界面活性,在临界胶束浓度(critical micelle concentration,CMC)为40–100 mg/L时,能将水的表面张力由72.8 mN/m降至40–30 mN/m[6]。槐糖脂的亲水亲油平衡值(hydrophile lipophilic balance,HLB)为10–13,可用作水包油型乳液的乳化剂。同时,槐糖脂拥有良好的耐盐特性和热稳定性[7]。
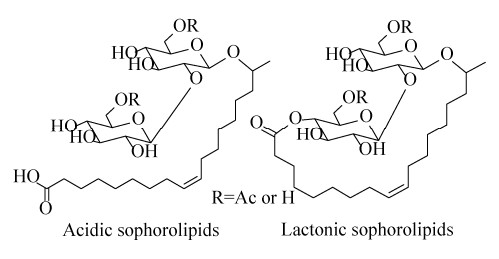 |
| 图 1 两大类槐糖脂的化学结构通式 Figure 1 Structure of sophorolipids. Left: acidic form; right: lactonic form. |
| 图选项 |
除了表现出良好的乳化、分散、增溶、自聚合等表面活性外,槐糖脂还具有抑菌、抗癌、抗炎、抗病毒、杀精等生物活性[1, 6-7]。然而,结构不同的槐糖脂在生物活性方面表现出相应的差异。内酯型相比于酸型槐糖脂而言,具有更显著的抑菌活性和抗癌活性[6];而酸型槐糖脂在抗病毒活性以及精子固定化性能上更具优势[8];同时,随着槐糖脂分子乙酰化程度的增强,其生物活性呈现上升的趋势[9]。此外,酸型槐糖脂的疏水脂肪酸链尾部包含-COOH基团(图 1),从而使其具备独特的pH敏感性和自聚合性能[10],而酸型槐糖脂的开环结构也为其在高附加值领域,尤其是生物医药领域的改性修饰提供了便利[11]。因此,为了发挥不同结构槐糖脂独特的性能优势,槐糖脂的分离纯化开始受到人们的广泛关注。本文将系统地综述近年来槐糖脂的发酵生产以及分离纯化相关的研究现状,并对相关研究进行展望,旨在促进槐糖脂的市场化发展。
1 槐糖脂的发酵生产研究现状 1.1 槐糖脂的生产菌株及其合成作用
1.1.1 野生菌株: 槐糖脂自1961年被首次发现后,因其卓越的表面活性和生物活性以及利用可再生资源产出的环境友好型生产方式引起了各界的广泛关注。在过去的几十年中,有关槐糖脂的研究报告日益增加,不断有关于新型生产菌株的文献报道,如表 1所示,概述了生产槐糖脂野生菌株的类型以及所产槐糖脂的主要结构。
表 1. 天然槐糖脂生产菌株种类及产物结构信息 Table 1. Wild sophorolipid-producing strains and their main type of products
| Microorganism | Strains origin | Main components |
| Candida apicola[2, 12] | Sow thistle petals, Canada | Mono- and nonacetylated lactonic form |
| Pseudohyphozyma bogoriensis[13-14] | Leaf surface of Randia malleifera, Indonesia | C22 diacetylated acidic form, OH at C13 |
| Candida floricola[15-16] | Dandelion and azalea, Japan | C18:1 diacetylated acidic form (ω-1) |
| Candida batistae[17] | Larva of digger bee, Brasil | C18:1 acidic form (ω) |
| Wickerhamiella domercqiae*[18] | Oil-containing wastewater | C18:1 diacetylated lactonic form (ω-1) |
| Wickerhamiella anomalus[19] | Thai fermented food | C18:1 /C20 SLs |
| Candida riodocensis[12] | Pollen and nectar of solitary bee | C18:1 diacetylated acidic and monoacetylated lactonic forms |
| Candida stellata[12] | Wine grapes, Germany | C18:1 diacetylated acidic form |
| Candida kuoi[12, 20] | Grape juice | C18:1 diacetylated acidic and monoacetylated lactonic forms |
| Candida rugosa[21] | Petroleum hydrocarbon-contaminated soil, India | C18:1 monoacetylated lactonic forms |
| Rhodotorula mucilaginosa[21] | Petroleum hydrocarbon-contaminated soil, India | C18:1 diacetylated acidic form |
| Candida tropicalis[22] | Petroleum hydrocarbon-contaminated site, India | C20:4 monoacetylated lactonic form |
| Candida albicans[23] | Ocean university of China | C18:1 diacetylated lactonic form |
| Cyberlindnera samutprakarnensis[24] | Cosmetic industrial waste | C18:0 nonacetylated lactonic and C16:0 diacetylated lactonic forms (ω-1) |
| Cryptococcus sp. VITGBN2[25] | Wastewater effluent, India | C18:1 diacetylated acidic form |
| Lachancea thermotolerans[26] | Gut of Apis mellifera, Iran | Acidic and lactonic forms mixture |
| Rhodotorula babjevae[27-28] | Agricultural field, India | C11:0/C13:1 nonacetylated acidic, C13:1/C15:3/C16:0/C18:2 nonacetylated lactonic and C18:0 diacetylated lactonic forms |
| Starmerella bombicola[29] | Bumblebee honey, Canada | C18:1 diacetylated lactonic form (ω-1) |
| *: W. domercqiae was identified as S. bombicola in recent years[1]. | ||
表选项
野生菌株分泌的槐糖脂可分为酸型和内酯型两种类型,又以内酯型为主,而脂肪酸链与糖原的连接则主要在ω和ω-1位点。其中,菌株Pseudohyphozyma bogoriensis和Candida kuoi的槐糖脂产物具有其独特之处。P. bogoriensis所产槐糖脂为22碳的二乙酰化酸型槐糖脂,脂肪酸链在C13位点处羟基化与糖原连接,与传统槐糖脂结构(ω或ω-1连接)截然不同[14](图 2-A)。C. kuoi等除生产常见的槐糖脂混合物外,还生产槐糖脂聚合产物[30],即一个酸型槐糖脂分子的羧基与另一个乙酰化的槐糖或是酸型槐糖脂在C4″位置通过酯键连接起来(图 2-B)。
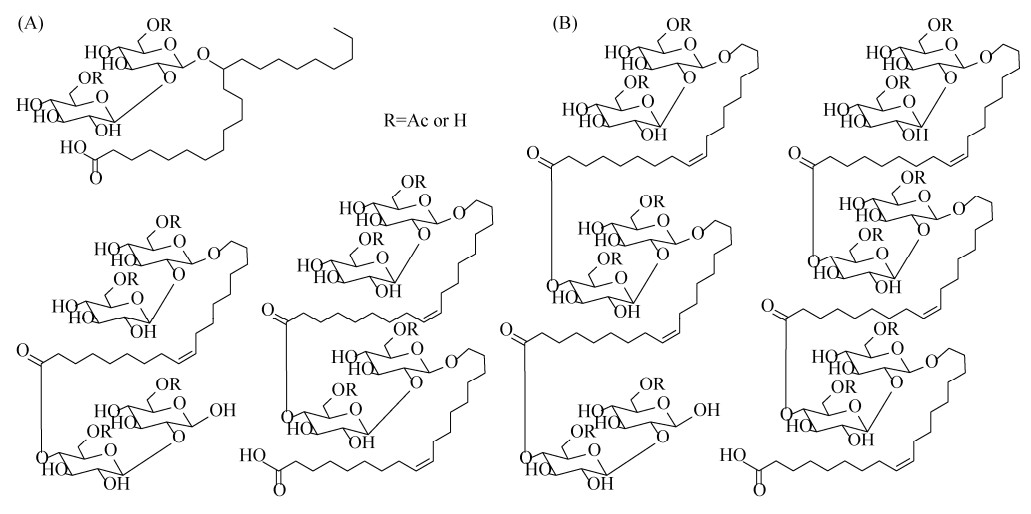 |
| 图 2 槐糖脂结构 Figure 2 Structure of sophorolipids. A: C22:0 sophorolipid[14]; B: Di- and trimeric sophorolipids produced by C. kuoi[30]. |
| 图选项 |
Starmerella bombicola是已知槐糖脂生产菌株中研究最多、产量最高、也是应用最广泛的野生菌株,又名Torulopsis bombicola或Candida bombicola,由Spencer等[31]从大黄蜂蜂蜜中筛选获得。通过系统地敲除菌株槐糖脂基因簇中负责槐糖脂合成的基因,已经明确了S. bombicola合成槐糖脂的生化途径,如图 3所示[1, 6-7]。
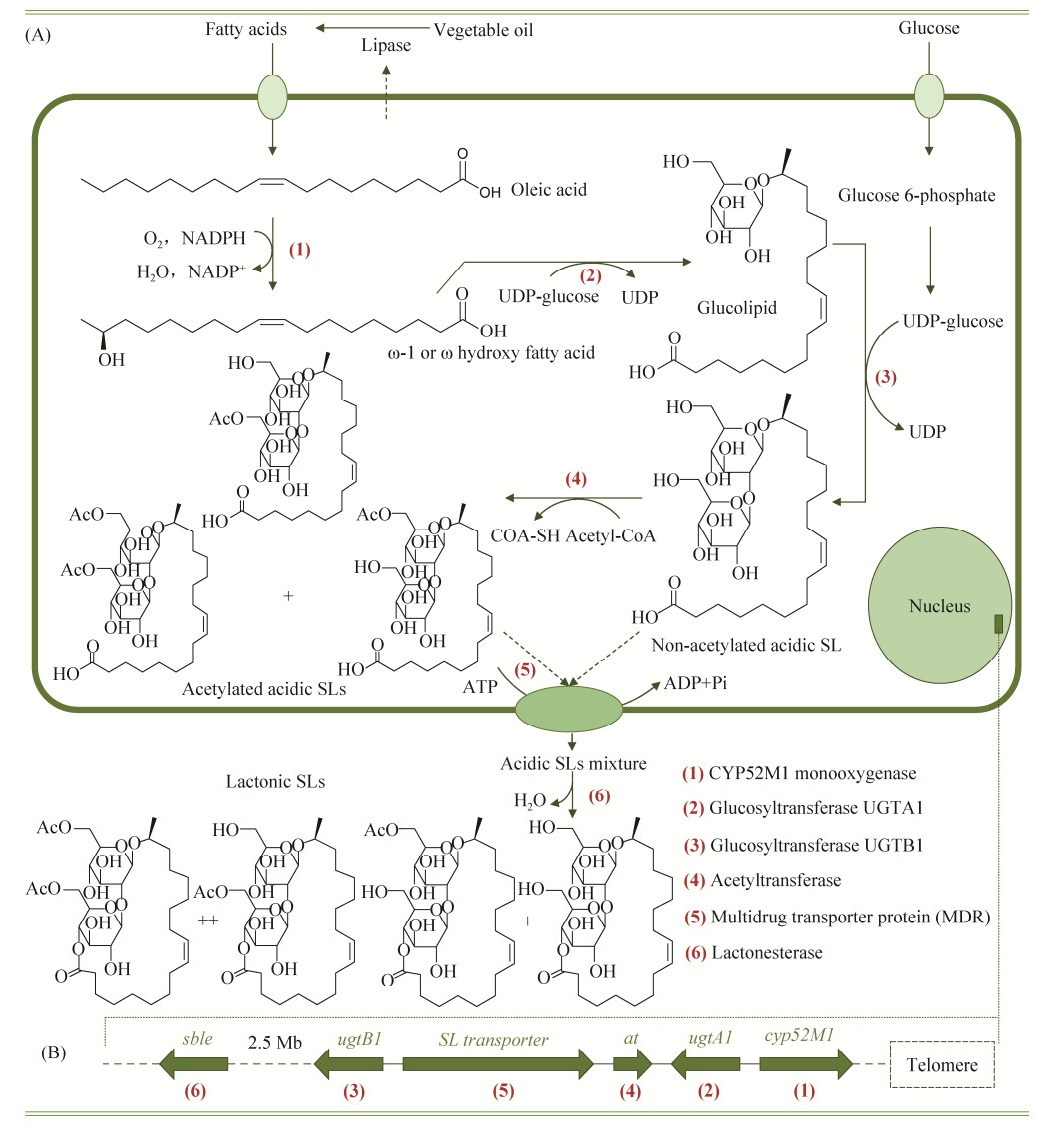 |
| 图 3 槐糖脂生物合成 Figure 3 Biosynthesis of sophorolipids[1, 6-7]. A: sophorolipids biosynthetic pathway; B: sophorolipids biosynthetic-related genes in S. bombicola. |
| 图选项 |
以葡萄糖和植物油为碳源时,植物油在酵母所产脂肪酶的作用下分解生成脂肪酸,这些脂肪酸通常含有16或18个碳原子以及1个或2个不饱和键。脂肪酸链进入细胞后在CYP52M1细胞色素P450单加氧酶的催化作用下在ω或是ω-1位置接入羟基生成羟基脂肪酸。与此同时,胞内C6′磷酸化的葡萄糖在UDP-葡萄糖焦磷酸化酶的催化作用下生成二磷酸尿苷葡萄糖(UDP-葡萄糖)。随后,在葡萄糖基转移酶UGTA1的作用下,已活化的葡萄糖(UDP-葡萄糖)的C1′接至羟基脂肪酸的羟基位置,生成葡萄糖脂;另一个活化葡萄糖的C1′在葡萄糖基转移酶UGTB1的催化作用下与葡萄糖脂的C2′相连从而生成无乙酰化的酸型槐糖脂。接着,无乙酰化的酸型槐糖脂在乙酰转移酶的作用下在C6′及C6″处随机乙酰化,生成单/双乙酰化酸型槐糖脂混合物。酸型槐糖脂混合物经MDR转运蛋白代谢至胞外,在胞外内酯酶的作用下,脂肪酸链的羧基端与糖原的C4" 连接,但也有少部分是在C6′或C6″处形成酯环[7],以合成内酯型槐糖脂混合物。
当在槐糖脂生产过程中仅提供亲水碳源(葡萄糖)时,葡萄糖糖酵解生成丙酮酸,丙酮酸经丙酮酸脱氢酶系作用生成乙酰辅酶A用以从头合成槐糖脂生产所需的脂肪酸[1];而当在槐糖脂发酵生产过程中仅以疏水物质(植物油)作为碳源时,脂肪酸经过β-氧化为细胞自身生存提供物质和能量的同时,也通过糖异生途径生成槐糖脂合成所需的葡萄糖[7]。
1.1.2 S. bombicola诱变菌株及基因工程菌株: 目前,野生型S. bombicola生产槐糖脂的产量可达400 g/L以上[32],但研究者仍期望使用菌株改造技术以获得更高的产量。Ichihara等[1]的专利报道S. bombicola的?pxa1、?pxa2和?fox2定向突变体的槐糖脂产量为野生型菌株的360%。然而,在某些应用领域和地区,转基因生物(genetically modified organisms,GMOs)产品的生产和销售受到限制[33],因此,传统的诱变育种仍然是获得性能优异菌株不可或缺的方法,同时高通量筛选技术的快速发展极大地增加了高性能突变体的可能性[34]。常压室温等离子体(atmospheric pressure and room temperature plasma,ARTP)技术是近几十年发展起来的一种全细胞诱变技术,它比化学诱变剂或紫外线辐射具有更大的DNA损伤和更高的突变率[35],被应用于S. bombicola菌株诱变以提高槐糖脂产量。ARTP技术孵育的突变体的槐糖脂产量为野生菌株的115%–140%[36-38],同时产物中酸型和内酯型槐糖脂的比例也随之改变。Ma等报道的突变体A6-9的内酯型槐糖脂产量为野生菌株的174%,A2-8的酸型产物产量为原来的152%[38]。同时,ARTP技术和化学诱变剂的联用能使突变体的高产性能得到显著提升,Chen和Tian等[39]联用ARTP技术和NaNO2诱变剂获得的Ncbio 5突变体的产量、产率及碳源转化率为野生菌株的126%、127%和135%。此外,通过低能离子束注入随机诱变技术[40]获得的W. domercqiae (后被鉴定为S. bombicola[1])最佳正向突变菌株的槐糖脂产量为野生型的184%,达135 g/L。
除提升槐糖脂的产量外,随着槐糖脂应用领域的不断拓展,具有特定结构或功能的槐糖脂已成为新的目标。因此,研究者们利用基因工程技术对S.bombicola进行了改造,如表 2所示。
表 2. S. bombicola工程菌株及槐糖脂产物 Table 2. Engineered S. bombicola strains and SLs products
| Engineered strains | Purpose | Main component | Yield/(g/L) |
| Δmfe-2[41] | To obtain 20-HETE | 20-HETE SLs | 19 |
| Δat[42] | To obtain SLs without acetyl groups | Nonacetylated lactonic form | 5 |
| oe sble[5] | To obtain lactonic SLs | Diacetylated lactonic form | 139 |
| Δsble[5] | To obtain acidic SLs | Acidic form mixture | 124 |
| ?at?sble[43] | To obtain SLs independent on pH | nsBola form | 63 |
| ?at?sble?fao1[43] | sBola form | 20 | |
| ?fao1[44] | To identify the function of fatty alcohol oxidase FAO1 | Alkyl SLs and Bola form | 27 |
表选项
Van Bogaert等[41]通过敲除参与脂肪酸β-氧化的多功能酶2型(mfe-2)的编码基因,使得所提供碳源花生四烯酸(二十碳四烯酸)无法转化为C16–C18脂肪酸,从而合成得到ω-C20:4槐糖脂(图 4-A),再通过酸水解途径获得目的产物20-羟基二十碳四稀酸(20-hydroxyeicosatetraenoic acid,20-HETE)。Saerens等[42]为了获得分子结构中不含有乙酰基团的槐糖脂产物,将负责编码乙酰转移酶的at基因敲除,但该措施使得槐糖脂的总产量下降了84.8%。Roelants等[5]通过过表达或敲除表达内酯酶的sble基因,实现了内酯型槐糖脂或酸型槐糖脂的归一化生产。Van Renterghem等[43]为获得不受环境pH影响的槐糖脂产物,通过选择性的敲除基因[at sble]和[at sble fao1],分别获得了非对称Bola型槐糖脂(non-symmetrical bolaform SLs,nsBola SLs)(图 4-C)和对称Bola型槐糖脂(symmetrical bolaform SLs,sBola SLs)(图 4-D)。Takahashi等[44]通过敲除编码脂肪醇氧化酶的基因,使得S. bombicola突变体无法将十四烷醇转化为脂肪酸,从而合成获得了烷基槐糖脂(图 4-B)和Bola型槐糖脂(图 4-E)。
 |
| 图 4 部分S. bombicola工程菌株生产槐糖脂的结构 Figure 4 Structure of sophorolipids produced by engineered S. bombicola strains. A: 20-Hydroxyeicosatetraenoic acidic sophorolipid[41]; B: alkyl sophorolipids[44]; C: non-symmetrical bola-form sophorolipid[43]; D: symmetrical bola-form sophorolipid[43]; E: bola-form sophorolipid[44]. |
| 图选项 |
1.2 槐糖脂的发酵工艺 S. bombicola作为槐糖脂的最佳生产者,其发酵生产相关研究十分广泛,尤其是在发酵培养条件的优化和新型发酵工艺的开发方面,大量研究者进行了广泛的探索。
1.2.1 发酵参数优化: (1) 培养基成分。碳源:S. bombicola发酵过程中,单一的亲水性底物(如葡萄糖)或疏水性底物(如植物油)均可作为碳源用于生产槐糖脂,但两者混合使用时,槐糖脂的产量更高。由于S. bombicola具有较强的耐渗透能力,发酵培养基的初始糖浓度通常可在100 g/L及以上。疏水性底物的成分最终会影响到槐糖脂产物的化学结构(脂肪酸链的长度及不饱和键数量),考虑到槐糖脂生化合成途径中单加氧酶CYP52M1进行脂肪酸羟基化衍生时对底物的亲和程度,油酸(C18:1)是最佳选择。为获得高的槐糖脂产量,同时考虑到生产成本,富含油酸的向日葵油(75%)和菜籽油(65%)通常用作槐糖脂发酵的疏水型碳源[43]。
氮源:酵母提取物是槐糖脂发酵生产过程中最常用的有机氮源,它富含含氮有机物、非含氮有机物、金属离子和维生素,对酵母细胞的生长和槐糖脂的合成具有重要影响。用尿素或蛋白胨替代酵母提取物时,不利于生物量的积累和槐糖脂的生产。但需要谨慎选择酵母提取物的使用剂量,低浓度酵母提取物(1 g/L)下的发酵使得生物量短时间内就累积到稳定期,有利于槐糖脂的生产;而高浓度的酵母提取物(10 g/L)则会延长微生物的生长期,使得发酵过程偏向于生物量的累积[34, 45]。
金属离子:金属离子对槐糖脂的生产起着重要作用,更准确地说是对参与槐糖脂生化合成酶的产生起着关键作用。例如:Fe2+是保持CYP52M1细胞色素P450单加氧酶高效性的必不可少的金属离子;Mg2+可能是葡萄糖转移酶UGTA1和UGTB1的辅助因子[46]。在实际生产中,添加Fe2+能促进酸型槐糖脂的合成,而Mg2+有利于内酯型槐糖脂的合成,此外添加一定量的Cu2+也有利于槐糖脂总产量的提升[47]。但某些金属离子,如Ni2+的使用对槐糖脂的生产往往带来负面影响[48]。
(2) 操作参数。pH:在S. bombicola的指数生长期,发酵液的pH会急剧下降,最终将维持在2.2左右。Gobbert等[49]测定了发酵pH为2.4–7.0槐糖脂的产量,发现当发酵液的pH维持在3.5时,槐糖脂的产量最高。因此,在后续的槐糖脂生产研究中,pH 3.5也是研究者们常用的发酵参数之一[50-52]。同时,较低的发酵液pH和槐糖脂本身具备的抗微生物能力能够保护发酵过程免受污染,使得长时间的发酵生产成为可能。
温度:S. bombicola的最佳生长温度是28.8 ℃ (national collection of yeast cultures,UK)。Gobbert等[49]指出槐糖脂合成的最佳温度是21 ℃。但在实际发酵生产过程中,槐糖脂生产中使用的温度一般均为25 ℃或30 ℃。
(3) 生理参数。生物量:在槐糖脂发酵过程中维持高浓度的生物量是获得高产量槐糖脂的必要条件。Gao等[53]使用15倍浓度的YM培养基(葡萄糖浓度除外)作为S. bombicola的培养基质,使得发酵罐中生物量迅速增长至最高值,是常规培养的6倍左右。随后向发酵罐中流加葡萄糖和菜籽油,槐糖脂产率达到了3.7 g/(L·h),为常规培养条件下的1.5–5.8倍,是现有报道中的最高产率。
溶解氧:在槐糖脂的整个发酵过程中,氧的供应非常重要,酵母细胞在指数生长过程中对氧限制非常敏感,良好的通气条件对槐糖脂的生产十分重要[7]。同时,槐糖脂生化合成的第一步,即脂肪酸的羟基化衍生过程中,CYP52M1细胞色素P450单加氧酶的催化反应需要使用氧分子(图 3)。Guilmanov等[54]通过摇瓶实验研究了通气量对槐糖脂产量的影响,发现氧的传递效率为50–80 mmol/L O2/(L·h)时,槐糖脂产量达到最大值。
1.2.2 流加发酵工艺: 流加发酵(fed-batch fermentation)是槐糖脂生产过程中最常用的发酵工艺之一。相比于传统的批次发酵工艺,流加发酵中槐糖脂的产量显著提升,其中又以碳源(如:葡萄糖、植物油和脂肪酸酯等)的流加为主[9, 43, 52-53, 72]。Davila等[50]发现,在亲水性碳源(葡萄糖)供应充足的条件下,流加脂肪酸酯等疏水碳源更有利于提高槐糖脂的产量,流加菜籽油酯(脂肪酸甲/乙醇酯)与流加菜籽油(脂肪酸甘油酯)相比,可将槐糖脂产量从255 g/L提升至340 g/L。然而,油脂酯类衍生物的价格昂贵,且在代谢途径中易于产生的甲醇/乙醇,使得槐糖脂的生产过程变得危险,因此未得到广泛的应用。另一种新颖的两步流加-批次发酵方法被Daniel等[55]报道,他们发现S. bombicola无法消耗乳清废液中的乳糖,故而在第一步发酵中利用Cryptococcus curvatus ATCC 20509分解乳糖,随后破碎细胞释放胞内油脂(single-cell oil,SCO)并得到细胞碎片,使用这种菌体破碎液作为第二步的发酵原料并在后续发酵过程中流加菜籽油,使得槐糖脂的产量达到了422 g/L。但同样的工艺条件下(使用C. curvatus菌体破碎液作为第二阶段的发酵原料),不流加菜籽油仅使用葡萄糖作为第二阶段发酵生产的碳源,槐糖脂产量低至12 g/L,产率仅为0.06 g/(L·h)[73]。可见,无论在批次发酵还是在流加发酵过程中,油脂对槐糖脂的高产具有重要影响。
槐糖脂发酵生产过程中,原料(植物油脂和葡萄糖)成本占总生产成本的10%–30%[74],因此,降低原料的成本有利于进一步降低槐糖脂的生产成本。近年来,大量的研究致力于使用农工业废弃物(如:糖蜜、秸秆、甘蔗渣、煎炸废油、厨余废物等)作为碳源进行槐糖脂生产[56-61]。为了进一步提升这些废弃物的使用效率,往往需要对其进行预处理,包括:酸碱水解[61, 75]、酶水解[57, 66]等。此外,在以这些废弃物为原料的发酵过程中流加传统的底物,如葡萄糖、大豆油、油酸等可进一步提升槐糖脂的产量。例如:Kaur等[57]通过向厨余废物水解液中流加葡萄糖和油酸将槐糖脂产量提高至原来的3.3倍。
虽然研究者们在槐糖脂的流加发酵工艺生产中取得了卓越的成效,但流加发酵工艺仍然具有不足之处。与批次发酵相同,流加发酵在微生物接种后的2d内几乎没有槐糖脂产出,这将降低整个发酵过程中的生产效率;流加发酵过程中的高产率状态往往无法维持,槐糖脂的生产阶段一般维持200h左右就不得不因为发酵液黏度过高而终止;发酵批次的切换使得发酵设备长时间停止运行,后续需要清洁、灭菌等,大大增加了运营成本[64]。为了降低发酵批次切换的频率,进一步减少前期准备时间,提升槐糖脂的产率,研究者引入了循环发酵的概念[76],并开发了半连续发酵工艺和连续发酵工艺[62, 64],有效地提升了设备的利用率。
1.2.3 半连续发酵工艺: 在半连续发酵工艺中,反应器中的产物累积到一定浓度后,部分富含产物的发酵液将被排出进入下游分离工序,然后新鲜的培养基加入反应器中,在补充营养物质的同时还有助于降低发酵液的粘度,有利于发酵的进行,如此循环往复,直至槐糖脂产量开始大幅度下降时发酵终止。Marchal等[62]进行了7个周期的循环发酵试验,反应器初始装液量为2 L,疏水碳源(菜籽油乙酯)的流加速率为2.1 g/h。每完成1个发酵周期(96 h),将移出一定量发酵液至反应器中剩余发酵液体积为1 L,随后加入1 L的新鲜培养基,开始新一批次的发酵培养,连续7个切换周期完成后,总计发酵时长672 h。最佳情况下(第7周期),槐糖脂产率可达2.34 g/(L·h),碳源转化率高达0.74。半连续发酵工艺的开发能有效缩短批次切换过程中所需的辅助时间,降低槐糖脂的生产成本,同时能在一定程度上降低产物的抑制效应并缓解发酵过程中的泡沫问题。但半连续发酵工艺仍存在不足之处,生产过程中发酵液的外排导致了生物量的损失,这需要额外的时间和物质能量来使细胞重新生长至稳定期(时间较批次发酵短),然后才能重新开始槐糖脂的生产。另外,工业规模上进行间歇性地取出发酵液和补充新鲜培养基存在操作困难,易染菌等风险。因此,研究者们对发酵工艺的优化进行了进一步探索。
1.2.4 连续发酵工艺: 为了减少生物量在循环发酵过程中的损失,研究者们开发了连续发酵工艺。在连续发酵工艺中,酵母细胞保留在反应器内,而产物可随发酵液流出而实现分离。因此,连续发酵也称“产物原位分离”发酵,这在一定程度上降低了产物抑制效应,同时也避免有害代谢废物的积累。该发酵工艺中,如何高效快速地保留(回收)菌体细胞显得尤为重要。Palme等[63]将超声波强化沉降(ultrasonic enhanced settling,USE)技术用于槐糖脂发酵过程中菌体的原位分离(图 5-A),在不影响细胞活力的前提下,细胞分离率最高可达99%,但存在槐糖脂回收率较低(约10%)等问题。为了提高槐糖脂的回收率,Dolman等[64]设计了一个独立的重力分离单元,开发了一种新颖的槐糖脂连续发酵生产工艺(图 5-B),并且能适应槐糖脂相密度的变化。该工艺在顶部分离情况下能够连续运行42 d,共分离获得623 g槐糖脂。作者认为槐糖脂相位于反应器的顶部或底部取决于发酵液中葡萄糖浓度是否超过50 g/L,但另有****报道发酵液中疏水底物含量的多少才是决定槐糖脂相位置的决定因素[65-66]。Liu等[65]首次报道了基于泡沫浮选分离的槐糖脂连续发酵工艺(图 5-C),通过调控体系中疏水底物与槐糖脂的比例,联用两级分离器,短时间内即可回收75%的槐糖脂。整个发酵工艺运行221 h后共获得777 g槐糖脂,回收率高达91%,生物量损失控制在1%以下,与批次发酵相比,产量提高了41%。Wang等[66]利用餐饮泔水的酶水解物作为底物,并进行葡萄糖和油酸的流加,利用分液漏斗作为简易分离器,开发了利用废弃物生产槐糖脂的连续发酵工艺(图 5-D)。取样静置1–2 min确定反应器内槐糖脂已累积至可沉淀水平,将全部发酵液泵入分离单元,静置10–15 min,通过控制管道的开关,将槐糖脂相排出发酵系统,而上部含微生物的发酵液泵回反应器内,流加底物后进行新一轮发酵。整个发酵过程(480 h)几乎没有生物量损失,槐糖脂的回收率高达93%,槐糖脂产率相比于(流加-)批次发酵提升了2–6倍。连续发酵工艺能够在最大程度上保留(回收)菌体细胞,实现槐糖脂的高效生产。但与半连续发酵相同,工业规模上的连续发酵工艺存在操作困难,易染菌等风险。
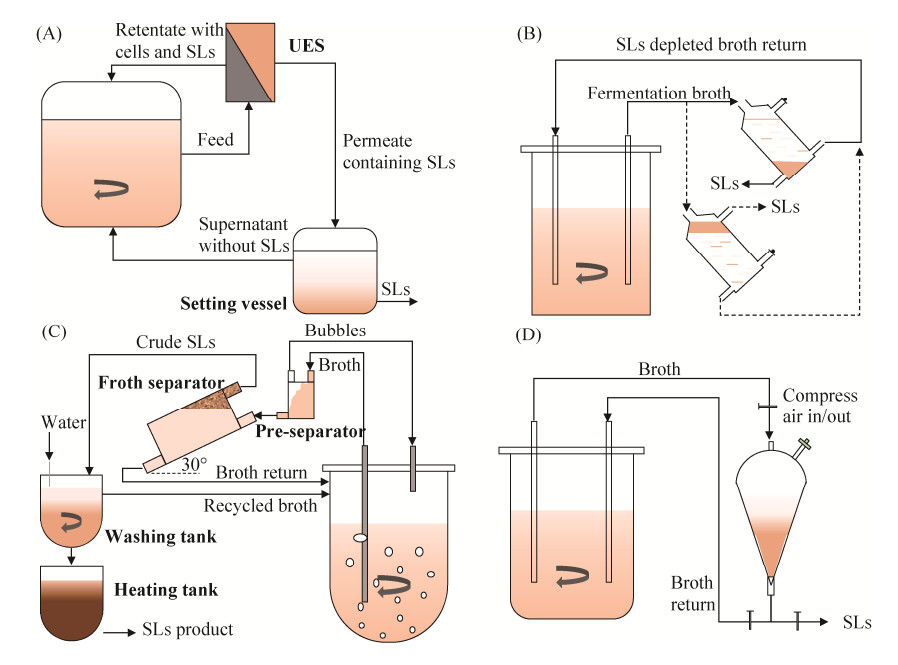 |
| 图 5 槐糖脂连续发酵生产工艺 Figure 5 Continuous fermentation process of sophorolipids production. A: in situ cell separation by applying ultrasonic enhanced setting (UES)[63]; B: gravity separation process adapted to density change of sophorolipids phase[64]; C: foam flotation separation process of sophorolipids[65]; D: gravity separation process of sophorolipids with funnel as simple separator[66]. |
| 图选项 |
泡沫控制一直是生物表面活性剂发酵生产过程中的难点[32],Wang等[66]指出,疏水性底物(油酸)的加入可有效消除槐糖脂发酵过程中产生的泡沫。但在发酵中后期,泡沫量增大,必须向反应器中加入较多的油酸才能维持发酵工艺的正常运行,由此导致分离单元中槐糖脂相无法聚集,只能进一步加大油酸的投入使得槐糖脂相聚集在分离器的上部得以分离,也因此造成了油酸的浪费。为了解决泡沫问题对槐糖脂发酵的困扰,固态发酵工艺的研究引起了众多****的兴趣。
1.2.5 固态发酵工艺: 与传统的沉浸式液体发酵相比,固态发酵可避免泡沫问题的产生[69],尤其是在生物表面活性剂的发酵过程中,槐糖脂固态发酵工艺的研究几乎全部以废弃物基质作为底物。Jiménez-Pe?alver等[69]在以冬化油饼和甜菜糖蜜作为底物的固态发酵工艺研究中发现,对基质进行间歇搅拌能够使槐糖脂的产量提升31%。同一课题组后续构建了以聚氨酯泡沫(polyurethane foams,PUF)为支撑,以硬脂酸和糖蜜为碳源的固态发酵工艺[71],通过优化PUF基床的比表面积和持水能力等发酵参数,获得了最佳槐糖脂产量0.211 g/g。然而,固态发酵工艺也不可避免地存在不足之处,例如:生物反应器的设计、发酵参数的监测以及槐糖脂的提取方式等。Jiménez-Pe?alver课题组发现固态发酵槐糖脂产量与微生物的累积耗氧量呈线性关系[69, 71],线性系数由具体的发酵工艺条件决定,这意味着有望实现槐糖脂固态发酵生产产量的在线检测。
虽然废弃物可以大量低价购得,利用废弃物取代传统基质作为发酵原料能够降低槐糖脂的生产成本。然而,实际上利用废弃物作为发酵原料会使得槐糖脂的成本增加。与传统发酵底物相比,废弃物生产槐糖脂的效率更低(表 3),需要投入更多的原料、机械运转、人工费用以获得等量的槐糖脂产物,同时废弃物的预处理成本也需要核算加入槐糖脂的生产成本中。现已有餐厨废物生产槐糖脂的计算机仿真经济分析报道[77],但该仿真评估依然与实际生产状况相去甚远,仅能作为参考。总而言之,以废物基质作为发酵原料,使得槐糖脂的生产朝着可循环的生物经济发展是可行的,但它们目前无法与高效的传统基质发酵工艺相竞争。针对废物基质设计改造更为强健、高产的菌株,使得废物基质“真正”取代传统基质成为可能。
表 3. 不同发酵工艺下的槐糖脂生产效率 Table 3. Production efficiency of sophorolipids under different fermentation processes
| Fed substrates | Fermentation scale | Yield/(g/L) | Productivity/ [g/(L·h)] | Conversion ratio */(g/g) | Quantitative methods |
| (Fed) Batch Fermentation | |||||
| Glucose, rapeseed esters[50] | 4 L | 340 | 2.10 | 0.65 | HPLC-ELSD |
| Glucose, rapeseed oil[53] | 10 L | >200 | 3.70 | 0.66 | Anthrone colorimetry |
| Glucose, SCO, rapeseed oil[55] | 3 L | 422 | 0.80 | 0.84 | Gravimetric |
| Sugarcane molasses, soybean oil[56] | 3 L | 60 | 0.31 | 0.60 | Gravimetric |
| Food waste hydrolysates[57] | 2 L | 28.2 | 0.39 | 0.26 | HPLC-ELSD |
| Glucose, sunflower oil refinery waste[58] | 5 L | 51.5 | 0.27 | 0.26 | Gravimetric |
| Rice bran, glucose, cottonseed oil[59] | 7 L | 131.5 | 0.67 | 0.44 | RP-HPLC |
| Soy molasses, oleic acid[60] | 12 L | 75 | 0.44 | 0.13 | Gravimetric |
| Glucose, tallow fatty acid[48] | Shake flask | 120 | 0.50 | 0.42 | TLC |
| Corn stover hydrolysates, yellow grease[61] | 3 L | 52.1 | 0.31 | 0.34 | Gravimetric |
| Food waste hydrolysates, glucose, oleic acid[57] | 2 L | 92.8 | 1.00 | 0.33 | HPLC-ELSD |
| Semi-continuous Fermentation | |||||
| Glucose, rapeseed esters[62] | 4 L | 266 (Cycle average) | 1.38 (Cycle average) | 0.43 (Cycle average) | Anthrone colorimetry |
| Continuous Fermentation | |||||
| Glucose, sunflower oil[63] | 10 L | 8.0 | 0.34 | 0.15 | TLC semi-quantitative |
| Glucose, rapeseed oil[64] | 3 L | 623 | 0.61 | 0.47 | Gravimetric |
| Glucose, rapeseed oil[65] | 5 L | 342 | 1.55 | 0.43 | Gravimetric |
| Food waste hydrolysates, glucose, oleic acid[66] | 2 L | 1719.7 | 2.39 | 0.73 | HPLC-ELSD |
| Solid-state Fermentation | |||||
| Fed substrates | Incubation time/h | Yield /(g/g) | |||
| Sunflower oil cake, motor oil[67] | 384 | 0.32 | |||
| Sunflower oil cake, soybean oil[68] | 384 | 0.40 | |||
| Winterization oil cake, sugar beet molasses[69] | 192 | 0.24 | |||
| Safflower oil cake, soybean oil[70] | 336 | 0.48 | |||
| Stearic acid, sugar beet molasses[71] | 384 | 0.21 | |||
*: 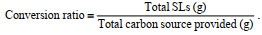 | |||||
表选项
2 槐糖脂的分离纯化研究现状 结构不同的槐糖脂具有各自独特的应用前景[6],为了充分发挥具有不同结构槐糖脂性能的优越性,分离获得特定类型的产物具有重要意义。然而,槐糖脂的下游处理过程中,产品的纯化成本占据总生产成本的60%–80%[1]。因此,为促进槐糖脂的商业化应用,研究开发高效低成本的槐糖脂分离纯化工艺十分重要。本章节将详细讨论近年来报道的槐糖脂分离纯化工艺及技术(表 4)。
表 4. 槐糖脂分离纯化工艺的分离效率及优缺点 Table 4. The comparison of separation efficiency by different processes
| Separation techniques | SL products | Recovery/% | Purity/% | Advantages | Disadvantages |
| Gravity[66] | Mixture | 93 | n.a. | Easy operation, high recovery rate, large-scale application | Low purity |
| Foam flotation[65] | Mixture | 91 | n.a. | Operating easily, high recovery rate, large-scale application | Low purity |
| Pentanol and Soxhlet extraction[78] | SL-COOH | ≤90 | >95 mol% | High purity, high recovery rate | Possible by-product formation |
| Double silica filtration[78] | SL-COOH | ≤60 | >92 mol% | Quick and easy operation | Highly toxic solvent, impurities retained |
| Preparative HPLC[18] | C18:1 diacetylated lactonic form | n.a. | 100 | 100% purity, single molecular structure product | Low instrument load, expensive |
| Crystallization (ethanol solution)[79] | acetylated lactonic form | 40 | >90 | High purity | Low recovery rate, long time |
| Crystallization (PBS solution)[80] | Diacetylated lactonic form | 99.2 | 98.9 | High purity, high recovery rate | Initial product content more than 90% |
| Crystallization (water solution)[5] | Diacetylated lactonic form | 95 | 97 | High purity, high recovery rate, large-scale application | For S. bombicola engineering strain products |
| Crystallization (mixed solvent)[78] | SL-COOH | ≤80 | 100 | High purity, high recovery rate | Long time, energy consumption, no water in system |
| Crystallization (ethyl acetate solvent)[81] | C22:0 monoacetylated acidic form | n.a. | >95 | High purity | Long time, energy consumption |
| Membrane filtration follow dialysis[5] | Acidic forms | 45 | 98 | High purity | Low recovery rate, membrane fouling |
| Membrane filtration follow dialysis[43] | Bola form | 65 | 95 | High purity | Low recovery rate, membrane fouling |
| Stepwise solvent extractions[82] | SL-COOH/SL-ester | 50/79 | 100 | High purity, universality | Need a lot of organic solvents |
| n.a.: not available. | |||||
表选项
2.1 重力分离 槐糖脂的密度比发酵液的密度大,到了发酵后期,大部分槐糖脂沉降在反应器底部,利用重力分离的原理可分离获得槐糖脂[64, 66, 82]。但仍有一部分槐糖脂溶解在发酵液中,为提升重力分离的回收率,加热能够促使发酵液中槐糖脂的沉淀[72, 82]。一般来说,所需的槐糖脂纯度取决于预期的应用,重力分离所得槐糖脂含有大量水分,并含有少量植物油、脂肪酸、色素等杂质,是槐糖脂的粗提物。这类粗品槐糖脂常被应用于对产品纯度要求不高的环境治理、石油回收等领域[83-84]。
2.2 泡沫浮选 泡沫分离是生物表面活性剂生产常用的分离技术之一,surfactin和鼠李糖脂已有通过泡沫浮选工艺进行产物分离[1]的报道。槐糖脂由于起泡性差且大部分产物自发沉降的特性无法通过泡沫浮选进行分离。Liu等[65]首次报道了槐糖脂的泡沫浮选分离工艺(图 5-C),通过调节发酵罐中菜籽油和槐糖脂的比例使槐糖脂相的密度减小,槐糖脂在短时间内不会自发沉降,该现象也被Wang等[66]报道。富含空气和含油槐糖脂的发酵液被泵入两级联用泡沫分离器中,在气泡的裹挟下,含油槐糖脂以泡沫的形式浮在发酵液表面,分离后进入下一个工作单元。泡沫分离器能在12min内分离获得75%的槐糖脂,整个生产工艺的槐糖脂分离率高达91%,但所得产物含油8%–12%,是槐糖脂的粗提物。
2.3 溶剂萃取 溶剂萃取法是生物表面活性剂分离工艺中普遍使用的方法。在实验室规模上,萃取槐糖脂最常用的有机溶剂是乙酸乙酯。用等体积的乙酸乙酯萃取槐糖脂发酵液2–3次,离心收集有机相,将乙酸乙酯旋转蒸发后可获得黄色槐糖脂粗品,再用正己烷洗涤粗品以去除油脂、脂肪酸等疏水性杂质,所得固体放入真空干燥箱中过夜。萃取法常用于槐糖脂重量法定量分析[65, 73],但萃取法所得槐糖脂仍然含有水分,疏水性杂质也无法完全去除,这往往会高估槐糖脂的产量。经典萃取法(乙酸乙酯为萃取剂)不具备选择性,由该方法纯化的槐糖脂仍然是成分复杂的混合物,无法实现不同类型槐糖脂的分离。
为了获得单一类型的槐糖脂,研究者们通常会使用化学法对槐糖脂混合物进行“预处理”,将结构复杂多样的混合物转变为某一类产物。Rau等[85]和Baccile等[78]将槐糖脂混合物碱水解,解开酯环并去除乙酰基团,使混合物转化为单一类型的去乙酰化酸型槐糖脂(SL-COOH),并用正戊醇萃取产物,通过索氏提取器进一步去除产物中的杂质[78],但所得酸型槐糖脂中仍含有亲水性杂质。近来,作者通过溶剂逐级萃取纯化技术分别获得了纯度极高的酸型和内酯型槐糖脂[82]。槐糖脂混合物在碱性甲醇溶液中发生反应获得SL-COOH,SL-COOH在酸性甲醇溶液中又转变为内酯型槐糖脂(SL-ester)。后续使用乙酸乙酯环己烷体系,乙酸乙酯甲醇体系和二氯甲烷对两类槐糖脂进行逐级萃取纯化,回收了约50%的酸型和80%的内酯型槐糖脂[82]。萃取法分离纯化槐糖脂操作简单,周期较短,能够快速制备产物,但需要使用大量的有机溶剂,并且无法直接进行酸型和内酯型槐糖脂的分离,需要借助化学修饰等预处理手段。
2.4 层析技术 柱层析是有机化合物分离纯化最常用最有效的技术,它也被应用到槐糖脂的分离纯化中。用于柱层析分离纯化的样品往往是已经通过溶剂萃取技术去除了大部分杂质的槐糖脂粗品。粗提物溶于有机溶剂并装填在硅胶柱上端,随后使用混合溶剂对硅胶柱进行梯度洗脱,收集不同阶段的层析液可获得分离产物。槐糖脂柱层析最为常用的有机溶剂体系是三氯甲烷-甲醇体系[86],三氯甲烷-丙酮溶剂体系也被报道可用于槐糖脂的柱层析分离[87]。柱层析技术能够获得高纯度的槐糖脂产物,但由于槐糖脂的结构过于复杂多样,并且每种结构间的差异较小,故而单一结构槐糖脂通过柱层析技术进行分离较为困难。同时,层析柱荷载低、层析柱装填难、产物回收率较低、回收周期长且需要使用大量有毒溶剂(如三氯甲烷)都使得槐糖脂柱层析分离技术难以大规模应用。为了克服上述技术难点,Baccile等[78]报道了一种无需硅胶柱装填的硅胶过滤纯化方法。通过交替使用亲水的60硅胶和疏水的C18硅胶分别除去SL-COOH中的亲水性和疏水性杂质,产物一直保留在流动的有机相中而被获得。该方法与传统硅胶柱层析相比,无需装填硅胶柱,纯化过程方便快捷,槐糖脂荷载量大(几十克),产物回收率较高(约60%),但在所得产品中会保留较多的杂质。
薄层层析(thin layer chromatography,TLC)也是槐糖脂常用的分离纯化技术之一。但该技术的应用并不是为了获得纯化产物,而是为了对槐糖脂进行定性分析[63]或是作为质谱分析的前端分离手段[84]。槐糖脂TLC分析时最常用的展开剂体系是氯仿:甲醇:水=65:15:2 (V/V/V),在展开后的硅胶板上喷洒硫酸-蒽酮溶液并于110 ℃加热5 min可观察到产物的显色[82]。
当对槐糖脂的抗癌活性、炎症反应等医用潜力进行深入探究时,槐糖脂的纯度和分离度要求将进一步提高。制备型高效液相色谱通常用于分离获得单一结构的槐糖脂纯净物[18]。与柱层析相似,制备型色谱的样品荷载率低,分离纯化成本高昂。
2.5 低温结晶 结晶法主要是针对在水中溶解度较低的乙酰化内酯型槐糖脂的分离纯化方法。早在20世纪60年代,就已有从槐糖脂乙醇溶液中低温结晶获得内酯型产物的报道[79]。为提升产物的回收率,Hu等[80]改进了此方法,在低温下用pH 6.5的磷酸缓冲液洗涤槐糖脂混合物以除去大部分酸型产物,随后在65 ℃用洗涤后的槐糖脂配置饱和溶液,再降温使得内酯型产物析出,成功回收99%以上内酯型槐糖脂。但该方法要求混合物中内酯型产物的含量大于90%,酸型杂质超载将降低产品的纯度及回收率。Roelants等[5]针对内酯酶过表达基因工程菌株生产的槐糖脂(99%二乙酰化内酯型)设计了多级热水洗涤-低温结晶的产物回收方法,回收率高达95%,产物纯度为97%。除内酯型槐糖脂外,部分酸型产物也能通过结晶法进行纯化。Baccile等[78]通过调节溶剂的极性,使得SL-COOH晶体在–18 ℃环境下析出。最近,Solaiman等[81]报道了一种结构独特的单乙酰化酸型槐糖脂(图 2-A)的低温结晶分离。产物的乙酸乙酯萃取液在–20– –18 ℃冷藏18 h以上,可析出晶体。低温结晶法获得的产物纯度高,但由于维持低温环境(4 ℃或–18 ℃)需要耗费大量能源,并且结晶过程需要较长时间,实际应用的可行性较低。
2.6 膜过滤 膜过滤分离纯化法适用于水溶性较好的酸型和Bola型槐糖脂。Roelants等[5]将酸型槐糖脂去细胞发酵液通过两级膜过滤进行分离纯化,第一步用30 kDa的超滤膜除去多糖、蛋白质和残留的菌体碎片,并将滤液透析5次,可回收78%的产物;第二步,用10 kDa的超滤膜去除第一步产物中的小分子杂质(糖、盐、小蛋白、多肽等),并将滞留物透析4次以除去色素杂质,该步骤的产物回收率为58%。Van Renterghem等[43]采用同样的两级膜过滤分离方法对含有2个亲水槐糖基的Bola型槐糖脂(图 4-C)进行纯化,回收了65%的产物。然而,超滤膜的价格昂贵,且使用过程中存在严重的膜污染问题(由于蛋白导致的),这也限制了该技术在实际中的应用。
综上,槐糖脂的分离纯化方法种类多样,但大多难以直接应用于大规模的槐糖脂制备,大型精密设备的配备以及能源的消耗会导致生产成本居高不下。为降低槐糖脂的生产成本,推进槐糖脂的市场化应用,高效率低成本的分离纯化工艺仍有待进一步探索。
3 展望 近年来,对槐糖脂应用研究的报道层出不穷,槐糖脂在石油回收、环境保护、药物开发等诸多领域表现出良好的应用前景。目前,槐糖脂纯品(纯度97%以上)和槐糖脂浓缩物(78%含量)的市场价格分别估计为38460美元/t和25640美元/t[77],远高于常规的化学表面活性剂。虽然槐糖脂的发酵生产水平高于其它的生物表面活性剂(surfactin、RHA和MELs等),但其产率和碳源转化率还有待提高,对半连续切换工艺和连续发酵工艺进行优化有望实现这一目的,例如:借助构建高密度细胞培养工艺,可显著提升槐糖脂产率;同时优化亲、疏水性碳源的添加比例,可进一步提升产物产率及底物转化率。此外,构建或选育高效利用工农业废弃物生产槐糖脂的工程菌株,提升其发酵工艺生产水平也可进一步降低槐糖脂生产成本,例如:联合使用ARTP技术和其它诱变技术并结合高通量筛选技术,有望在获得高产量菌株的同时实现单一种类槐糖脂的生产(酸型或内酯型)。对于槐糖脂的分离纯化工艺,使用化学修饰手段或借助特定生物酶催化手段将产物的复杂成分归一化,并结合溶剂萃取技术可实现高效的分离纯化目的,有望降低其生产成本。而该分离技术的完善也有助于进一步开发槐糖脂高附加值领域(如化妆品、制药、新材料等)的应用,提升其与化学表面活性剂的竞争,有助于加速其产业化应用。
References
| [1] | Roelants S, Solaiman DKY, Ashby RD, Lodens S, Van Renterghem L, Soetaert W. Production and applications of sophorolipids. //Hayes DG, Solaiman DKY, Ashby RD. Biobased Surfactants. 2nd ed. Urbana: AOCS Press, 2019: 65-119. |
| [2] | Gorin PAJ, Spencer JFT, Tulloch AP. Hydroxy fatty acid glycosides of sophorose from Torulopsis magnoliae. Canadian Journal of Chemistry, 1961, 39(4): 846-855. DOI:10.1139/v61-104 |
| [3] | Tulloch AP, Spencer JFT. Fermentation of long-chain compounds by Torulopsis apicola. IV. products from esters and hydrocarbons with 14 and 15 carbon atoms and from methyl palmitoleate. Canadian Journal of Chemistry, 1968, 46(9): 1523-1528. DOI:10.1139/v68-249 |
| [4] | Claus S, Van Bogaert INA. Sophorolipid production by yeasts: a critical review of the literature and suggestions for future research. Applied Microbiology and Biotechnology, 2017, 101(21): 7811-7821. DOI:10.1007/s00253-017-8519-7 |
| [5] | Roelants SLKW, Ciesielska K, De Maeseneire SL, Moens H, Everaert B, Verweire S, Denon Q, Vanlerberghe B, Van Bogaert INA, Van Der Meeren P, Devreese B, Soetaert W. Towards the industrialization of new biosurfactants: biotechnological opportunities for the lactone esterase gene from Starmerella bombicola. Biotechnology and Bioengineering, 2016, 113(3): 550-559. DOI:10.1002/bit.25815 |
| [6] | Delbeke EIP, Movsisyan M, Van Geem KM, Stevens CV. Chemical and enzymatic modification of sophorolipids. Green Chemistry, 2016, 18(1): 76-104. DOI:10.1039/C5GC02187A |
| [7] | Van Bogaert INA, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ. Microbial production and application of sophorolipids. Applied Microbiology and Biotechnology, 2007, 76(1): 23-34. DOI:10.1007/s00253-007-0988-7 |
| [8] | Shah V, Doncel GF, Seyoum T, Eaton KM, Zalenskaya I, Hagver R, Azim A, Gross R. Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrobial Agents and Chemotherapy, 2005, 49(10): 4093-4100. DOI:10.1128/AAC.49.10.4093-4100.2005 |
| [9] | Otto RT, Daniel HJ, Pekin G, Müller-Decker K, Fürstenberger G, Reuss M, Syldatk C. Production of sophorolipids from whey. Applied Microbiology and Biotechnology, 1999, 52(4): 495-501. DOI:10.1007/s002530051551 |
| [10] | Baccile N, Babonneau F, Jestin J, Pehau-Arnaudet G, Van Bogaert I. Unusual, pH-induced, self-assembly of sophorolipid biosurfactants. ACS Nano, 2012, 6(6): 4763-4776. DOI:10.1021/nn204911k |
| [11] | Delbeke EIP, Roman BI, Marin GB, Van Geem KM, Stevens CV. A new class of antimicrobial biosurfactants: quaternary ammonium sophorolipids. Green Chemistry, 2015, 17(6): 3373-3377. DOI:10.1039/C5GC00120J |
| [12] | Kurtzman CP, Price NPJ, Ray KJ, Kuo TM. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiology Letters, 2010, 311(2): 140-146. DOI:10.1111/j.1574-6968.2010.02082.x |
| [13] | Tulloch AP, Spencer JFT, Deinema MH. A new hydroxy fatty acid sophoroside from Candida bogoriensis. Canadian Journal of Chemistry, 1968, 46(3): 345-348. DOI:10.1139/v68-057 |
| [14] | Solaiman DKY, Ashby RD, Crocker NV. High-titer production and strong antimicrobial activity of sophorolipids from Rhodotorula bogoriensis. Biotechnology Progress, 2015, 31(4): 867-874. DOI:10.1002/btpr.2101 |
| [15] | Tokuoka K, Ishitani T, Goto S, Komagata K. Four new yeast species belonging to the genus Candida. The Journal of General and Applied Microbiology, 1987, 33(1): 1-10. DOI:10.2323/jgam.33.1 |
| [16] | Imura T, Masuda Y, Minamikawa H, Fukuoka T, Konishi M, Morita T, Sakai H, Abe M, Kitamoto D. Enzymatic conversion of diacetylated sophoroselipid into acetylated glucoselipid: surface-active properties of novel bolaform biosurfactants. Journal of Oleo Science, 2010, 59(9): 495-501. DOI:10.5650/jos.59.495 |
| [17] | Rosa CA, Viana EM, Martins RP, Antonini Y, Lachance MA. Candida batistae, a new yeast species associated with solitary digger nesting bees in Brazil. Mycologia, 1999, 91(3): 428-433. DOI:10.1080/00275514.1999.12061036 |
| [18] | Chen J, Song X, Zhang H, Qu YB. Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme and Microbial Technology, 2006, 39(3): 501-506. DOI:10.1016/j.enzmictec.2005.12.022 |
| [19] | Thaniyavarn J, Chianguthai T, Sangvanich P, Roongsawang N, Washio K, Morikawa M, Thaniyavarn S. Production of sophorolipid biosurfactant by Pichia anomala. Bioscience, Biotechnology, and Biochemistry, 2008, 72(8): 2061-2068. DOI:10.1271/bbb.80166 |
| [20] | Kurtzman CP. Candida kuoi sp. nov., an anamorphic species of the Starmerella yeast clade that synthesizes sophorolipids. International Journal of Systematic and Evolutionary Microbiology, 2012, 62(Pt_9): 2307-2311. DOI:10.1099/ijs.0.039479-0 |
| [21] | Chandran P, Das N. Characterization of sophorolipid biosurfactant produced by yeast species grown on diesel oil. International Journal of Engineering Science, 2011, 2: 63-71. |
| [22] | Chandran P, Das N. Role of sophorolipid biosurfactant in degradation of diesel oil by Candida tropicalis. Bioremediation Journal, 2012, 16(1): 19-30. DOI:10.1080/10889868.2011.628351 |
| [23] | Yang X, Zhu LQ, Xue CY, Chen Y, Qu L, Lu WY. Recovery of purified lactonic sophorolipids by spontaneous crystallization during the fermentation of sugarcane molasses with Candida albicans O-13-1. Enzyme and Microbial Technology, 2012, 51(6/7): 348-353. |
| [24] | Poomtien J, Thaniyavarn J, Pinphanichakarn P, Jindamorakot S, Morikawa M. Production and characterization of a biosurfactant from Cyberlindnera samutprakarnensis JP52T. Bioscience, Biotechnology, and Biochemistry, 2013, 77(12): 2362-2370. DOI:10.1271/bbb.130434 |
| [25] | Basak G, Das D, Das N. Dual role of acidic diacetate sophorolipid as biostabilizer for ZnO nanoparticle synthesis and biofunctionalizing agent against Salmonella enterica and Candida albicans. Journal of Microbiology and Biotechnology, 2014, 24(1): 87-96. DOI:10.4014/jmb.1307.07081 |
| [26] | Mousavi F, Beheshti-Maal K, Massah A. Production of sophorolipid from an identified current yeast, Lachancea thermotolerans BBMCZ7FA20, isolated from honey bee. Current Microbiology, 2015, 71(2): 303-310. DOI:10.1007/s00284-015-0841-7 |
| [27] | Sen S, Borah SN, Bora A, Deka S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microbial Cell Factories, 2017, 16(1): 95. DOI:10.1186/s12934-017-0711-z |
| [28] | Sen S, Borah SN, Kandimalla R, Bora A, Deka S. Sophorolipid biosurfactant can control cutaneous dermatophytosis caused by Trichophyton mentagrophytes. Frontiers in Microbiology, 2020, 11: 329. DOI:10.3389/fmicb.2020.00329 |
| [29] | Davila AM, Marchal R, Monin N, Vandecasteele JP. Identification and determination of individual sophorolipids in fermentation products by gradient elution high-performance liquid chromatography with evaporative light-scattering detection. Journal of Chromatography A, 1993, 648(1): 139-149. DOI:10.1016/0021-9673(93)83295-4 |
| [30] | Price NPJ, Ray KJ, Vermillion KE, Dunlap CA, Kurtzman CP. Structural characterization of novel sophorolipid biosurfactants from a newly identified species of Candida yeast. Carbohydrate Research, 2012, 348: 33-41. DOI:10.1016/j.carres.2011.07.016 |
| [31] | Spencer JF, Gorin PA, Tulloch AP. Torulopsis bombicola sp. n. Antonie Van Leeuwenhoek, 1970, 36(1): 129-133. DOI:10.1007/BF02069014 |
| [32] | Jiang JJ, Zu YQ, Li XY, Meng Q, Long XW. Recent progress towards industrial rhamnolipids fermentation: Process optimization and foam control. Bioresource Technology, 2020, 298: 122394. DOI:10.1016/j.biortech.2019.122394 |
| [33] | Bruetschy C. The EU regulatory framework on genetically modified organisms (GMOs). Transgenic Research, 2019, 28(2): 169-174. |
| [34] | Van Renterghem L, Clicque H, Huyst A, Roelants SLKW, Soetaert W. Miniaturization of Starmerella bombicola fermentation for evaluation and increasing (novel) glycolipid production. Applied Microbiology and Biotechnology, 2019, 103(11): 4347-4362. DOI:10.1007/s00253-019-09766-3 |
| [35] | Ottenheim C, Nawrath M, Wu JC. Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): the latest development. Bioresources and Bioprocessing, 2018, 5(1): 1-14. DOI:10.1186/s40643-017-0187-z |
| [36] | Zhou G, Tian XW, Lin YM, Zhang SL, Chu J. Rational high-throughput system for screening of high sophorolipids-producing strains of Candida bombicola. Bioprocess and Biosystems Engineering, 2019, 42(4): 575-582. DOI:10.1007/s00449-018-02062-w |
| [37] | Lin YM, Chen Y, Li QH, Tian XW, Chu J. Rational high-throughput screening system for high sophorolipids production in Candida bombicola by co-utilizing glycerol and glucose capacity. Bioresources and Bioprocessing, 2019, 6(1): 1-9. DOI:10.1186/s40643-018-0235-3 |
| [38] | Ma XJ, Zhang HM, Lu XF, Han J, Zhu HX, Wang H, Yao RS. Mutant breeding of Starmerella bombicola by atmospheric and room-temperature plasma (ARTP) for improved production of specific or total sophorolipids. Bioprocess and Biosystems Engineering, 2020, 43(10): 1869-1883. DOI:10.1007/s00449-020-02377-7 |
| [39] | Chen Y, Tian XW, Li QH, Li Y, Chu J, Hang HF, Zhuang YP. Target-site directed rational high-throughput screening system for high sophorolipids production by Candida bombicola. Bioresource Technology, 2020, 315: 123856. DOI:10.1016/j.biortech.2020.123856 |
| [40] | Li H, Ma XJ, Shao LJ, Shen J, Song X. Enhancement of sophorolipid production of Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 by low-energy ion beam implantation. Applied Biochemistry and Biotechnology, 2012, 167(3): 510-523. DOI:10.1007/s12010-012-9664-1 |
| [41] | Van Bogaert I, Zhang GD, Yang J, Liu JY, Ye YH, Soetaert W, Hammock BD. Preparation of 20-HETE using multifunctional enzyme type 2-negative Starmerella bombicola. Journal of Lipid Research, 2013, 54(11): 3215-3219. DOI:10.1194/jlr.D042226 |
| [42] | Saerens KMJ, Saey L, Soetaert W. One-step production of unacetylated sophorolipids by an acetyltransferase negative Candida bombicola. Biotechnology and Bioengineering, 2011, 108(12): 2923-2931. DOI:10.1002/bit.23248 |
| [43] | Van Renterghem L, Roelants SLKW, Baccile N, Uyttersprot K, Taelman MC, Everaert B, Mincke S, Ledegen S, Debrouwer S, Scholtens K, Stevens C, Soetaert W. From lab to market: an integrated bioprocess design approach for new-to-nature biosurfactants produced by Starmerella bombicola. Biotechnology and Bioengineering, 2018, 115(5): 1195-1206. DOI:10.1002/bit.26539 |
| [44] | Takahashi F, Igarashi K, Hagihara H. Identification of the fatty alcohol oxidase FAO1 from Starmerella bombicola and improved novel glycolipids production in an FAO1 knockout mutant. Applied Microbiology and Biotechnology, 2016, 100(22): 9519-9528. DOI:10.1007/s00253-016-7702-6 |
| [45] | Casas J, García-Ochoa F. Sophorolipid production by Candida bombicola: medium composition and culture methods. Journal of Bioscience and Bioengineering, 1999, 88(5): 488-494. DOI:10.1016/S1389-1723(00)87664-1 |
| [46] | Saerens KMJ, Van Bogaert INA, Soetaert W. Characterization of sophorolipid biosynthetic enzymes from Starmerella bombicola. FEMS Yeast Research, 2015, 15(7): fov075. DOI:10.1093/femsyr/fov075 |
| [47] | Chen J, Zhang HR, Liu Y, Fu SM, Liu XL. Metal ions can affect the composition and production of sophorolipids by Wickerhamiella domercqiae Y2A CGMCC 3798. European Journal of Lipid Science and Technology, 2014, 116(11): 1505-1512. DOI:10.1002/ejlt.201300512 |
| [48] | Felse PA, Shah V, Chan J, Rao KJ, Gross RA. Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzyme and Microbial Technology, 2007, 40(2): 316-323. DOI:10.1016/j.enzmictec.2006.04.013 |
| [49] | G?bbert U, Lang S, Wagner F. Sophorose lipid formation by resting cells of Torulopsis bombicola. Biotechnology Letters, 1984, 6(4): 225-230. DOI:10.1007/BF00140041 |
| [50] | Davila AM, Marchal R, Vandecasteele JP. Sophorose lipid production from lipidic precursors: predictive evaluation of industrial substrates. Journal of Industrial Microbiology, 1994, 13(4): 249-257. DOI:10.1007/BF01569757 |
| [51] | Davila AM, Marchal R, Vandecasteele JP. Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Applied Microbiology and Biotechnology, 1997, 47(5): 496-501. DOI:10.1007/s002530050962 |
| [52] | Kim YB, Yun HS, Kim EK. Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture. Bioresource Technology, 2009, 100(23): 6028-6032. DOI:10.1016/j.biortech.2009.06.053 |
| [53] | Gao RJ, Falkeborg M, Xu XB, Guo Z. Production of sophorolipids with enhanced volumetric productivity by means of high cell density fermentation. Applied Microbiology and Biotechnology, 2013, 97(3): 1103-1111. DOI:10.1007/s00253-012-4399-z |
| [54] | Guilmanov V, Ballistreri A, Impallomeni G, Gross RA. Oxygen transfer rate and sophorose lipid production by Candida bombicola. Biotechnology and Bioengineering, 2002, 77(5): 489-494. DOI:10.1002/bit.10177 |
| [55] | Daniel HJ, Reuss M, Syldatk C. Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in a two stage fed batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnology Letters, 1998, 20(12): 1153-1156. DOI:10.1023/A:1005332605003 |
| [56] | Daverey A, Pakshirajan K. Kinetics of growth and enhanced sophorolipids production by Candida bombicola using a low-cost fermentative medium. Applied Biochemistry and Biotechnology, 2010, 160(7): 2090-2101. DOI:10.1007/s12010-009-8797-3 |
| [57] | Kaur G, Wang HM, To MH, Roelants SLKW, Soetaert W, Lin CSK. Efficient sophorolipids production using food waste. Journal of Cleaner Production, 2019, 232: 1-11. DOI:10.1016/j.jclepro.2019.05.326 |
| [58] | Jadhav JV, Pratap AP, Kale SB. Evaluation of sunflower oil refinery waste as feedstock for production of sophorolipid. Process Biochemistry, 2019, 78: 15-24. DOI:10.1016/j.procbio.2019.01.015 |
| [59] | Haque F, Sajid M, Cameotra SS, Battacharyya MS. Anti-biofilm activity of a sophorolipid-amphotericin B niosomal formulation against Candida albicans. Biofouling, 2017, 33(9): 768-779. DOI:10.1080/08927014.2017.1363191 |
| [60] | Solaiman DKY, Ashby RD, Zerkowski JA, Foglia TA. Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnology Letters, 2007, 29(9): 1341-1347. DOI:10.1007/s10529-007-9407-5 |
| [61] | Samad A, Zhang J, Chen D, Chen XW, Tucker M, Liang YN. Sweet sorghum bagasse and corn stover serving as substrates for producing sophorolipids. Journal of Industrial Microbiology & Biotechnology, 2017, 44(3): 353-362. |
| [62] | Remy M, Michel W, Bernard C. Process for the production of sophorolipids by cyclic fermentation with feed of fatty acid esters or oils. US: 5879913. 1997-10-17. |
| [63] | Palme O, Comanescu G, Stoineva I, Radel S, Benes E, Develter D, Wray V, Lang S. Sophorolipids from Candida bombicola: cell separation by ultrasonic particle manipulation. European Journal of Lipid Science and Technology, 2010, 112(6): 663-673. DOI:10.1002/ejlt.200900163 |
| [64] | Dolman BM, Kaisermann C, Martin PJ, Winterburn JB. Integrated sophorolipid production and gravity separation. Process Biochemistry, 2017, 54: 162-171. DOI:10.1016/j.procbio.2016.12.021 |
| [65] | Liu ZP, Tian XW, Chen Y, Lin YM, Mohsin A, Chu J. Efficient sophorolipids production via a novel in situ separation technology by Starmerella bombicola. Process Biochemistry, 2019, 81: 1-10. DOI:10.1016/j.procbio.2018.12.005 |
| [66] | Wang HM, Kaur G, To MH, Roelants SLKW, Patria RD, Soetaert W, Lin CSK. Efficient in situ separation design for long-term sophorolipids fermentation with high productivity. Journal of Cleaner Production, 2020, 246: 118995. DOI:10.1016/j.jclepro.2019.118995 |
| [67] | Rashad MM, Al-kashef AS, Nooman MU, Mahmoud AEE. Engco-utilization of motor oil waste and sunflower oil cake on the production of new sophorolipids by Candida bombicola NRRL Y-17069. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2014, 5(4): 1515-1528. |
| [68] | Rashad MM, Nooman MU, Ali MM, Al-Kashef AS, Mahmoud AE. Production, characterization and anticancer activity of Candida bombicola sophorolipids by means of solid state fermentation of sunflower oil cake and soybean oil. Grasas y Aceites, 2014, 65(2): e017. DOI:10.3989/gya.098413 |
| [69] | Jiménez-Pe?alver P, Gea T, Sánchez A, Font X. Production of sophorolipids from winterization oil cake by solid-state fermentation: optimization, monitoring and effect of mixing. Biochemical Engineering Journal, 2016, 115: 93-100. DOI:10.1016/j.bej.2016.08.006 |
| [70] | Nooman MU, Mahmoud MH, Al-kashef AS, Rashad MM. Hypocholesterolemic impact of newly isolated sophorolipids produced by microbial conversion of safflower oil cake in rats fed high-fat and cholesterol diet. Grasas y Aceites, 2017, 68(3): e212. DOI:10.3989/gya.0219171 |
| [71] | Jiménez-Pe?alver P, Castillejos M, Koh A, Gross R, Sánchez A, Font X, Gea T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. Journal of Cleaner Production, 2018, 172: 2735-2747. DOI:10.1016/j.jclepro.2017.11.138 |
| [72] | Delbeke EIP, Roelants SLKW, Matthijs N, Everaert B, Soetaert W, Coenye T, Van Geem KM, Stevens CV. Sophorolipid amine oxide production by a combination of fermentation scale-up and chemical modification. Industrial & Engineering Chemistry Research, 2016, 55(27): 7273-7281. |
| [73] | Daniel HJ, Otto RT, Binder M, Reuss M, Syldatk C. Production of sophorolipids from whey: development of a two-stage process with Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 using deproteinized whey concentrates as substrates. Applied Microbiology and Biotechnology, 1999, 51(1): 40-45. DOI:10.1007/s002530051360 |
| [74] | Mnif I, Ghribi D. Glycolipid biosurfactants: main properties and potential applications in agriculture and food industry. Journal of the Science of Food and Agriculture, 2016, 96(13): 4310-4320. DOI:10.1002/jsfa.7759 |
| [75] | Samad A, Zhang J, Chen D, Liang YN. Sophorolipid production from biomass hydrolysates. Applied Biochemistry and Biotechnology, 2015, 175(4): 2246-2257. DOI:10.1007/s12010-014-1425-x |
| [76] | McCaffrey WC, Cooper DG. Sophorolipids production by Candida bombicola using self-cycling fermentation. Journal of Fermentation and Bioengineering, 1995, 79(2): 146-151. DOI:10.1016/0922-338X(95)94082-3 |
| [77] | Wang HM, Tsang CW, To MH, Kaur G, Roelants SLKW, Stevens CV, Soetaert W, Lin CSK. Techno-economic evaluation of a biorefinery applying food waste for sophorolipid production - a case study for Hong Kong. Bioresource Technology, 2020, 303: 122852. DOI:10.1016/j.biortech.2020.122852 |
| [78] | Baccile N, Cuvier AS, Valotteau C, Van Bogaert INA. Practical methods to reduce impurities for gram-scale amounts of acidic sophorolipid biosurfactants. European Journal of Lipid Science and Technology, 2013, 115(12): 1404-1412. DOI:10.1002/ejlt.201300131 |
| [79] | Tulloch AP, Hill A, Spencer JFT. Structure and reactions of lactonic and acidic sophorosides of 17-hydroxyoctadecanoic acid. Canadian Journal of Chemistry, 1968, 46(21): 3337-3351. DOI:10.1139/v68-551 |
| [80] | Hu YM, Ju LK. Purification of lactonic sophorolipids by crystallization. Journal of Biotechnology, 2001, 87(3): 263-272. DOI:10.1016/S0168-1656(01)00248-6 |
| [81] | Solaiman DKY, Ashby RD, Nu?ez A, Crocker N. Low-temperature crystallization for separating monoacetylated long-chain sophorolipids: characterization of their surface-active and antimicrobial properties. Journal of Surfactants and Detergents, 2020, 23(3): 553-563. DOI:10.1002/jsde.12396 |
| [82] | Tang YJ, Ma QQ, Du YL, Ren L, Van Zyl LJ, Long XW. Efficient purification of sophorolipids via chemical modifications coupled with extractions and their potential applications as antibacterial agents. Separation and Purification Technology, 2020, 245: 116897. DOI:10.1016/j.seppur.2020.116897 |
| [83] | Xu QX, Liu XR, Wang DB, Liu YW, Wang QL, Ni BJ, Li XM, Yang Q, Li HL. Enhanced short-chain fatty acids production from waste activated sludge by sophorolipid: Performance, mechanism, and implication. Bioresource Technology, 2019, 284: 456-465. DOI:10.1016/j.biortech.2019.03.121 |
| [84] | Elshafie AE, Joshi SJ, Al-Wahaibi YM, Al-Bemani AS, Al-Bahry SN, Al-Maqbali D, Banat IM. Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in microbial enhanced oil recovery. Frontiers in Microbiology, 2015, 6: 1324. |
| [85] | Rau U, Heckmann R, Wray V, Lang S. Enzymatic conversion of a sophorolipid into a glucose lipid. Biotechnology Letters, 1999, 21(11): 973-977. DOI:10.1023/A:1005665222976 |
| [86] | Konishi M, Fukuoka T, Morita T, Imura T, Kitamoto D. Production of new types of sophorolipids by Candida batistae. Journal of Oleo Science, 2008, 57(6): 359-369. DOI:10.5650/jos.57.359 |
| [87] | Konishi M, Morita T, Fukuoka T, Imura T, Uemura S, Iwabuchi H, Kitamoto D. Selective production of acid-form sophorolipids from glycerol by Candida floricola. Journal of Oleo Science, 2017, 66(12): 1365-1373. DOI:10.5650/jos.ess17116 |
