李雅卓1, 李章2, 张继彪1, 付杰1,2


1. 复旦大学环境科学与工程系, 上海 200438;
2. 华中科技大学环境科学与工程学院, 湖北 武汉 430074
收稿日期:2020-03-20;修回日期:2020-05-16;网络出版日期:2020-05-26
基金项目:国家自然科学基金(91851110,41701541)
作者简介:付杰, 华中科技大学环境科学与工程学院教授、博导, 中国城镇供水排水协会青年工作者委员会常务委员, 中国医学装备协会医院水系统专业委员会"医院水系统研究中心"副主任。主要从事污染物环境行为与生态效应的研究, 致力于研究污染物在环境中的迁移转化规律及其与环境因子的相互作用、评估污染物的环境行为对地球化学过程的影响。近年来先后主持国家自然科学基金重大研究计划培育项目、青年科学基金等多项科研项目。在Environmental Scicence&Technology、Water Research等国际权威期刊发表SCI论文120余篇, 第一作者及通讯作者80余篇, ESI高被引论文5篇, 总引用>3200次, H指数33。获得湖北省自然科学三等奖、首届"安捷伦杯"中国化学快报环境化学青年科学家奖.
*通信作者:付杰, Tel/Fax:+86-21-31248907;E-mail:jiefu@fudan.edu.cn.
摘要:2010年深水地平线事故发生后,在被石油污染的墨西哥湾观察到大量的“海油雪”形成,海油雪的相关研究成为人们关注的焦点。海油雪是指石油、浮游植物、细菌黏液等组成的团聚物,能够将石油从海面沉降至海底,对石油的风化过程产生深远影响。因此,研究海油雪的形成机制和生态效应,对于深入认识海油雪在石油-海洋系统中的作用具有重要意义。本文从物理凝聚、微生物和石油分散剂三个方面对海油雪的形成机制展开探讨,分析了海油雪对石油风化、底栖生物毒性和其他污染物迁移转化的影响,并结合现有研究进行了展望。
关键词:海油雪凝聚胞外聚合物分散剂风化生物毒性
Formation mechanism and ecological effect of "marine oil snow"
Yazhuo Li1, Zhang Li2, Jibiao Zhang1, Jie Fu1,2


1. Department of Environmental Science and Engineering, Fudan University, Shanghai 200438, China;
2. School of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, Hubei Province, China
Received: 20 March 2020; Revised: 16 May 2020; Published online: 26 May 2020
*Corresponding author: Jie Fu, Tel/Fax: +86-21-31248907; E-mail: jiefu@fudan.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (91851110, 41701541)
Abstract: After the Deepwater Horizon accident in 2010, a large number of "marine oil snow" (MOS) were observed in the oil-polluted area of the Gulf of Mexico. MOS is a kind of agglomerate composed of oil, phytoplankton and bacterial slime, which can sink oil from the sea surface to the seafloor and has great influence to the weathering process of oil. Therefore, investigating the formation mechanism and ecological effect of marine oil snow is of great significance for further understanding the role of marine oil snow in the oil-ocean system. In this paper, the formation mechanism of MOS is discussed from three aspects of physical agglomeration, microorganism and oil dispersant, and the influences of MOS on oil weathering, benthos toxicity and migration and transformation of other pollutants are analyzed. Finally, future research directions are proposed.
Keywords: marine oil snowcoagulationextracellular polymer substancedispersantweatheringbiological toxicity
“海雪” (marine snow)是指海洋中肉眼可见的(> 0.5 mm)固体聚集物,其成分复杂包括矿物碎屑、细菌粘液、浮游生物、排泄物等[1-3]。因其无定形的形状,在海洋中下沉就像雪花飘落,所以称其为“海雪”[4]。海雪广泛分布于海洋中,一般丰度为0–100颗粒/L,沉降速度可达16–368 m/d[5]。海雪对海洋具有重要的作用,可概括为:(1)提供与水相相对隔离的微环境,为生物群落以及营养物质的分布提供了斑状性;(2)发挥纵向传递介质作用,加快颗粒物质垂直传输;(3)为浮游动物和鱼类提供重要食物来源。
在过去的几十年里,由于油轮事故、管道、钻井平台泄漏,导致大量石油被释放到海洋环境中,在受到石油污染的海域中还会形成包含石油组分的“海油雪” (marine oil snow,MOS)[6]。例如,在2010年“深水地平线” (DWH)漏油事件发生后,在石油钻井平台附近观察到大体积的海油雪絮状物(> 1 cm),经过1个月的漂浮后,这些絮状物沉降到海底[7]。海油雪形成对石油的吸附迁移、生物降解等风化过程具有重要影响。本文对海油雪形成机制及其生态效应展开综述。
1 海油雪形成机制 1.1 物化机制 海油雪形成的主要机制是物理凝聚,包括悬浮矿物颗粒、石油油滴和絮状物、微生物以及分泌物等的碰撞和粘附。驱动颗粒物碰撞的机制主要是海洋湍流剪切力,适当的湍流可以引起颗粒的悬浮并促进粒子碰撞[8]。本课题组通过辊台实验模拟了海油雪的形成,辊台的转动提供了足够的旋转湍流,使得粒子悬浮,增加了悬浮粒子的碰撞几率,促进了颗粒聚合[1]。悬浮颗粒在旋转罐子切面的运动轨迹呈圆形,受到流体阻力(FD) (小雷诺数Stokes公式1)和与重力-浮力(FG) (公式2)两种力的作用(图 1)[8]。
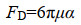 | 公式(1) |
 | 公式(2) |
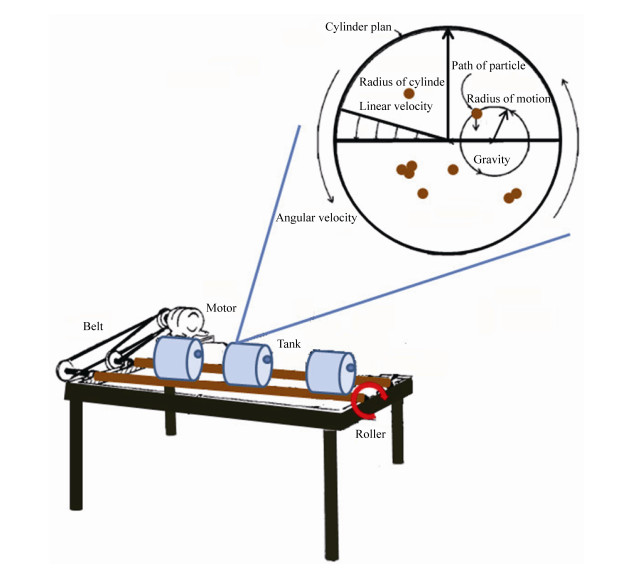 |
| 图 1 模拟海油雪形成的辊台和颗粒物的运动轨迹 Figure 1 Roller table for simulating MOS formation and the motion orbit of suspending particles in turbulence. |
| 图选项 |
μ是流体粘度,α是颗粒半径,V是颗粒体积,ρ和ρf是颗粒和流体的密度,g是重力加速度。
海洋系统中大多数粒子带负电荷,根据Derjaguin-Landau-Verwey-Overbeek (DLVO)理论,可以来解释这些带电粒子的碰撞和粘附。控制粒子的相互作用是范德华吸引力(WA)和静电斥力(ER),当碰撞能量克服最大能势达到第一最小值,聚沉发生,达到第二最小值时,絮凝发生[9]。非DLVO理论(如疏水力和路易斯酸碱作用)也在海油雪的形成中起重要作用。由于有机物质的存在,如石油烃、分散剂、胞外聚合物,增加了表面粘性,导致摩擦碰撞,比弹性碰撞更有效。因此,不仅形成了更多的海油雪,而且由于动能转化为内能而产生的热效应,使得絮凝体体积更大[1]。
根据碰撞理论、凝聚理论和湍流力学,我们可以推导出海油雪在湍流中体积增长的理论计算公式如下。悬浮粒子要聚合的第一步是两个粒子要相遇碰撞,粒子的碰撞率(E)可以由公式(3)表示。
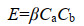 | 公式(3) |
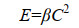 | 公式(4) |
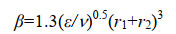 | 公式(5) |
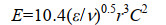 | 公式(6) |
 | 公式(7) |
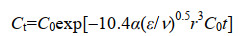 | 公式(8) |
 | 公式(9) |
1.2 微生物作用机制 目前普遍认为,微生物在海油雪形成过程中发挥了重要作用。许多微生物包括细菌、真菌与藻类[13-18]会分泌具有很强粘性的胞外聚合物(extracellular polymer substance,EPS),EPS与海洋颗粒相互作用可以大大增强其粘性系数α,从而促进海洋颗粒的快速聚合。EPS以纳米纤维的形式从细胞表面脱落[19],自组装成胶体大小的纳米凝胶[20],有时能形成肉眼可见的(μm-mm)透明胞外聚合颗粒(transparent exopolymer particles,TEP)[21]。TEP是粘性颗粒,可以为海洋颗粒聚合提供粘聚力和粘性基质[22]。从组成上看,EPS的主要成分是糖类、蛋白质、核酸,这些大分子物质可以通过絮凝和桥接作用引起颗粒聚集;主要官能团包括羧基、氨基和羟基,能够通过静电和路易斯酸碱作用引起颗粒聚集[1, 23]。
许多实验室模拟研究以及采集海油雪样品检测都证明了EPS在海雪和海油雪形成过程的重要作用。Shanks和Edmondson[24]首次进行辊台实验成功在实验室制造出了海雪聚集物,并发现细菌粘液(胞外多糖)在海雪聚集过程中发挥了至关重要的作用。Suja等[25]对海油雪进行显微镜染色观察,发现其成分主要为糖蛋白,与EPS的组成相一致,本课题组实验模拟了海油雪形成过程,发现添加石油后的4 d内,溶液中颗粒数从912±28减少到18±3,聚集率达到223.5/d,并且第2天时总细菌数与EPS分别达到(2.38±0.14)×104 cells/mL、2.59±0.17 mg/L,是不添加石油溶液的3.2倍和7倍[1]。Van等研究发现,从浮游植物中分离的细菌能够产生EPS,在无菌环境中不会产生EPS,表明与浮游植物相关的细菌生成了EPS,Passow等[26]研究发现,在没有石油存在时,Thalassiosira weissflogii和Dactyliosolen fragilissimus硅藻在几天内形成了1 mm大小的团聚物,石油的添加促进了EPS的产生,导致大粒径(10 mm)团聚体的快速形成,并且两种硅藻聚集物中分别有65%和50%的碳来自于石油。表明微生物活动对于海油雪的形成起着至关重要的作用。
在石油污染的海域,大量石油烃降解微生物可以在很短的时间内迅速占据优势[27]。能够代谢石油烃的主要是一些细菌,主要来自γ-变形菌纲(Gammaproteobacteria)和α-变形菌纲(Alphaproteobacteria) (图 2)。在富集的石油烃降解菌中也有许多能产生大量EPS的细菌,比如盐单胞菌(Halomonas)、交替单胞菌(Alteromonas)和假交替单胞菌(Pseudoalteromonas)[14]。石油的存在不仅为细菌的生长提供了额外的碳源和能源,导致更多EPS和黏液的生成[7]。同时细菌产生的EPS具有两亲性,很容易与疏水性底物(如碳氢化合物)相互作用,能够作为生物表面活性剂乳化石油,提高生物利用度,增强石油烃和悬浮粒子的相互作用[14, 18, 28-29]。
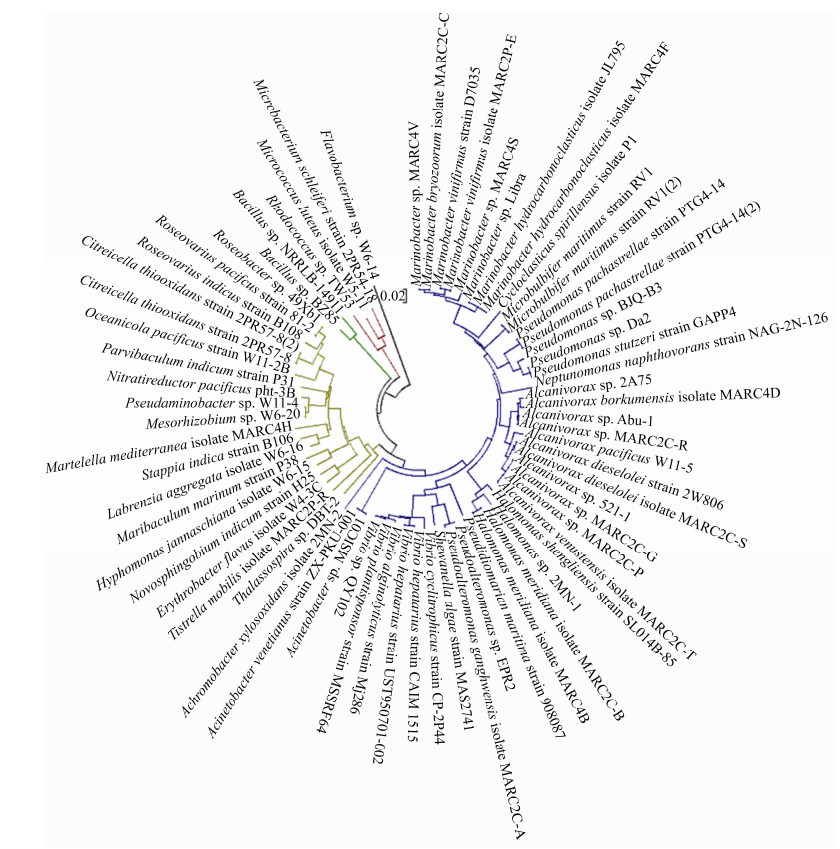 |
| 图 2 海洋石油烃降解细菌进化关系分析 Figure 2 Phylogenetic analysis of petroleum hydrocarbon degrading bacteria in oceans. |
| 图选项 |
1.3 石油分散剂的作用 石油分散剂是表面活性剂(阴离子和非离子表面活性剂)和有机溶剂的混合物,它可以通过降低表面张力将浮油分解成细小的液滴,从而加速石油在水柱中的分散,增强石油组分的溶解,提高石油的生物降解性[30-31]。几十年来,使用石油分散剂一直是缓解海上石油泄漏影响的关键措施[32-33]。在2010年的“深水地平线”漏油事件中,作为紧急措施,大约210万加仑的石油分散剂(Corexit EC9527A和Corexit EC9500A)施用于海面(140万加仑)和钻井口(77万加仑)[34-35]。大量石油分散剂进入海洋环境,对海洋生物和人类健康的造成了潜在的威胁,已经成为海洋环境科学研究的热点问题[36-37]。
大量研究表明分散剂的存在促进了石油组分在海油雪中的分配与富集。分散剂的加入降低了油与海水之间的界面张力,促进了石油在水体中的分散[38]。Ramachandran等[32]发现Corexit EC9500A与油比1:50时,能使油在水中的分散度提高68%。当分散剂浓度超过临界胶束浓度(critical micelle concentration,CMC)时,会导致更多小油滴的形成,增加石油与悬浮粒子的碰撞几率,促进海油雪的形成[32, 34]。石油分散剂还可促进悬浮粒子对石油组分的吸附,提高海油雪中石油的含量。本课题组的研究发现石油分散剂体系中,海油雪中正构烷烃(0.48 mg,9.2%)的含量几乎是纯油体系(0.17 mg,3.3%)的3倍[1]。Li等[39]研究了分散剂Corexit EC9500A的添加对海油雪中石油含量的影响,发现分散剂与石油比为1:50时,海油雪中石油含量提高了将近2倍。这是因为分散剂或表面活性剂可以被吸附到悬浮颗粒表面形成胶束或半胶束,通过增溶作用提高对疏水性石油烃化合物的吸收。
分散剂对于海油雪形成的影响存在争议,分散剂corexit中烃类化合物含量高达30%W/W,可以作为细菌的碳源和能源,刺激大量EPS的产生,促进海油雪的形成[13, 40]。例如,Suja等[25]研究发现,添加石油和分散剂后,海油雪在7 d内快速形成,4周后由初始的2–3 mm增加至2 cm,在仅添加分散剂时,3 d内形成了大的(0.5–3.0 cm)白色聚集体。本课题组研究发现,分散剂的添加导致溶液中颗粒数2 d内从934±99减少至18±4,聚集率223.5/d,并且14 d时的总细菌数和EPS分别是初始的15.3倍和115倍[1]。韩璐[41]研究发现,分散剂与石油比DOR在1:8–1:3时,分散剂的添加对海油雪的生成量有显著影响,分形维数和EPS含量均随投加量的增大先升高后下降,当DOR为1:5时,絮体的分形维数值达最大为1.5453,细菌分泌的EPS含量高,形成的海油雪最为密实。然而一些研究表明分散剂Corexit在一定程度上会减缓、减少甚至完全抑制海油雪的形成[26],可能是分散剂的存在影响了细菌分泌EPS的能力、分散了已经形成的凝胶[42]或通过选择分散剂而不是石油作为底物的细菌改变了海水中的微生物群落[43-45]。于洋[44]研究发现,分散剂的添加导致石油污染海水中的菌群从食碱菌科、海洋螺菌科、黄杆菌科、产碱菌科向红菌科、红螺菌科过渡。Hamdan等[46]研究表明,分散剂的存在显著降低了石油烃降解菌不动杆菌(Acinetobacter)和海杆菌(Marinobacter)的生长和生存能力。而Baelum等[47]则观察到Corexit对微生物生长没有负面影响。造成分散剂效果不同的原因可能与微生物群落、石油类型、海洋环境条件等因素有关,有待进一步澄清。
2 海油雪的生态效应 2.1 海油雪对石油组分风化的影响 进入海洋的石油组分将经历一系列的物理化学生物风化过程,包括吸附、生物降解、分散、溶解、乳化、蒸发和光降解等[37, 48-49]。据估算,2010年墨西哥湾漏油事故导致500万桶的原油进入海洋,其中大约14%的溢油通过海油雪沉降至沉积物中,沉淀面积达3200–24000 km2[50],沉积速率至少是漏油事件前后的4倍[51]。海油雪的形成导致石油从海面输送到海底,影响了石油污染在海洋生态系统中的分布和循环。
原油泄漏过程中,降解原油的细菌活性增加,Arnosti等[18]发现海油雪聚集物具有明显不同于周围海水的细菌群落组成,“深水地平线”石油泄漏期间从墨西哥湾收集的海油雪主要由石油降解细菌解环菌(Cycloclasticus)、海洋杆菌(Marinobacter)和产生EPS的盐单胞菌(Halomonas)组成,Ziervogel等[52]通过检测海油雪中的脂肪酶水解活性,发现与周围的海水介质相比,海油雪聚集体中的烃类降解率明显更高,表明海油雪为微生物群落的生存提供了基质,促进了石油的生物降解。Wirth等[53]对海油雪中石油组分进行分析,发现海油雪中C18/phytane的比例(1.3)低于悬浮油滴中相应的比例(1.8),Wozniak等[54]采用傅立叶变换离子回旋共振质谱(FT-ICR MS)技术对海油雪中石油组分进行分析,发现与原油相比,海油雪中的石油不饱和度DBE和含氧化合物含量较高,可能是细菌降解过程将脂肪烃、脂环烃和芳香烃氧化为醇、酮、醛、内酯、羧酸等的结果(图 3)[55-57]。以上结果证实了海油雪中微生物对于石油组分的降解,促进了石油组分的风化。
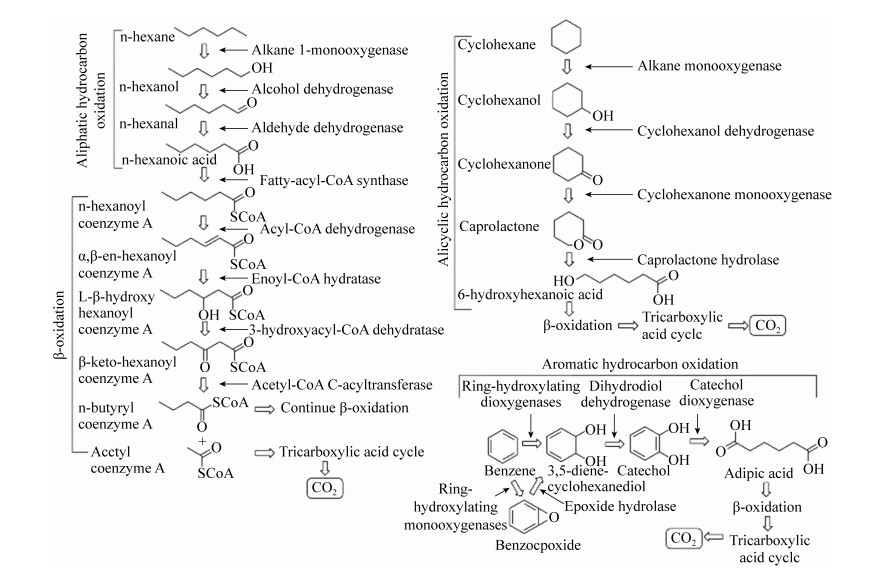 |
| 图 3 石油烃模型分子(正己烷、环己烷和苯)微生物降解示意图 Figure 3 Schematic diagram showing microbial degradation of petroleum hydrocarbon model molecules (n-hexane, cyclohexane and benzene). |
| 图选项 |
2.2 海油雪对底栖生物的毒性效应 海洋泄漏石油通过海油雪沉积和絮凝堆积(marine oil snow sedimentation and flocculent accumulation,MOSSFA)沉降到海底,对底栖生物造成了明显的负面影响[58],包括伤害生物个体、增加群落死亡率以及改变群落组成结构等。底栖生物一般是指生活在潮间带到海底表面和沉积物中的生物,种类多样且具有一系列的生态功能(营养循环、生物扰动和为更高的营养水平提供食物资源等),对海洋生态系统具有十分重要的生态学意义。
小型底栖生物中占主导地位的是线虫,其次为桡足类生物,由于小型底栖生物分布广泛、生活周期短、对环境变化反应迅速,因此常将底栖线虫和桡足类丰度比值(the nematode:copepod ratio,N/C)作为评估有机富集和化学污染的指标[59-60]。研究表明受石油泄漏污染严重地区,线虫数量上升,而桡足类生物由于对环境胁迫敏感,数量下降10倍,使得N/C值增加,小型底栖生物丰度增加,多样性下降。其中海油雪沉降导致的有机富集或生物本身对碳氢化合物的高耐受性是线虫数量上升的主要原因[61-62]。同时,MOSSFA事件后,海油雪的生物降解消耗氧气,海底有机物积累增加微生物的呼吸作用,使沉积物孔隙水中氧气减少,也会影响底栖生物[63]。实验室研究中也证实了人工海油雪会以极快的速度消耗氧气[64]。底栖有孔虫是一类单细胞原生生物,遍布全球海洋,通常被用作海洋石油污染的指标。2010年和2011年初,DWH事件后,距井口东北方向120 km的两处,底栖有孔虫的密度下降了80%–93%,原因有可能是海油雪沉积引起的缺氧环境导致有孔虫的生殖或死亡受到抑制[65]。2018年一项研究指出,大型底栖无脊椎动物在海雪作用下存活率降低[50]。
生物本身能动性决定了其是否能够逃脱不利的环境,底栖生物中固着生物对于石油泄漏受到的影响远远大于能动的物种。DWH事件后,离井口6 km处,90%的深水珊瑚群均发现有含油的絮凝物质,被海油雪覆盖的珊瑚表现出压力(如产生过多的粘液、硬结增大、组织丧失)和死亡的迹象,且暴露在高浓度海油雪的枝干至今尚未恢复[66-68]。珊瑚生长极其缓慢,寿命长,恢复速度缓慢,极易受到干扰,且一些鱼类和鲨鱼会将深海珊瑚用作产卵地,海油雪对珊瑚的影响可能会导致更大的生态系统威胁。同时,海油雪会影响底栖鱼类,海油雪沉积导致石油中所含多环芳烃浓度升高,其毒性会造成鱼类皮肤损伤以及多种免疫毒性反应,如生长受损、疾病易感性增加、幼虫存活率降低和净群体繁殖力降低[69-70]。
海雪作为由微小的死亡有机物和活有机体结合而成的固体聚集物,是许多浮游生物的食物来源,石油以海雪作为媒介,被摄食后,通过食物网在生物体内累积会增加高水平生物对石油的暴露风险[71]。综上,海油雪主要通过摄食、微生物活动、窒息、缺氧条件、食物网转移、鳃皮暴露或者生物累积产生免疫毒性等途径对于底栖生物产生影响,进而影响海洋生态系统。同时,海雪的生物降解会同石油生物降解竞争氧气,优先降解,导致石油降解缓慢,Ziervogel等[72]检测到MOSSFA事件后,沉积物中的微生物增长率呈现温和增长的趋势,因此作者推测,海油雪可能会在海底停留很长时间,海油雪的长期停留,对深海生态环境也存在长期的影响。
2.3 海油雪对其他污染物迁移转化的影响 海洋污染物除石油外,还有营养盐(以无机氮和活性磷酸盐为主)及重金属(铜、铅、镉、锌、砷、汞、铬)等其他污染物。海油雪对海洋中的其他污染物也存在一定的影响,在此我们以汞(mercury,Hg)为例展开阐述。Hg及其化合物是一类具有慢性剧毒的环境污染物,对生物的影响取决其于浓度、化学形态以及生物本身特征。Hg在水中的形态可能为单质汞、无机汞和有机汞3种,海水中由于Cl-浓度很高,主要存在形态为HgCl42-[73]。形成过程见公式(10)和(11)。
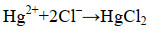 | 公式(10) |
 | 公式(11) |
 |
| 图 4 汞在微生物作用下转化示意图 Figure 4 Schematic diagram showing mercury transformation mediated by microorganisms in natural environments. |
| 图选项 |
DWH事件泄漏石油中含汞的浓度较低,且后续被水稀释,不会直接影响该水域的汞浓度,其对水体中汞形态转化的影响主要通过沉积物沉积,海底化学成分以及微生物活性改变等方面[77-79]。石油泄漏导致沉积速率增加,海油雪中的海雪对大多数离子都有较大的吸引力,从而提高水体中的汞向海底的沉积速率,使得水体中的汞通过颗粒沉降,滞留在沉积物中[80]。随后,新沉积的汞会同营养匮乏区域的有机物相结合,增加该地区生物网中汞的生物累积[81],以及同有机物中还原性硫位点结合形成络合作用极强的离子键,增加汞的迁移[82-83]。同时,汞为沉积物时,对水生生物毒性减弱,可能会被生物有机体还原成单质,蒸发到大气中(图 4)。对于微生物活性影响方面,汞和有机质的结合,会限制Hg2+对甲基化细菌的可用性,从而降低汞的生物积累[83]。
MOSSFA事件会导致海底氧化还原反应增强,从而影响海底微生物活动。许多SRB都可以参与到汞的还原、甲基化和去甲基化过程,但是研究表明只有一些有特定基因的SRB可以显著地甲基化汞,促进甲基汞的生成。Perrot等[81]研究了受石油污染区域方头鱼体内汞的浓度,在鱼体内发现了较低水平的甲基汞,由于在大多数鱼类,几乎所有的汞(95%以上)都是以甲基汞的形式出现,说明甲基汞的脱甲基和无机汞的还原程度均高于甲基汞的生成。摄食率低和健康状况不佳也会导致鱼体内汞的积累。DWH事件后,鱼类的饮食习惯也受到影响而改变,最近一项研究证明方头鱼,作为一类以底部沉积物为食的底栖鱼类,受DWH影响,生物量下降高达70%,且饥饿加剧[84]。MOSSFA导致的有机富集可以通过刺激微生物生长来促进甲基汞的形成,也可以通过络合减少无机汞的甲基化降低甲基汞的含量,同时络合作用会引起汞的迁移。总的来说,海油雪会降低甲基汞的生成,同时引起的食物网结构的变化,会影响鱼类的摄食策略降低甲基汞在鱼体内的积累,限制汞的生物利用度。
3 总结和展望 海油雪形成机制复杂,涉及到石油、分散剂、微生物之间的相互作用,石油的存在为微生物的生长提供了额外的碳源和能源,导致更多EPS的生成,同时微生物分泌的EPS能够乳化石油,较强粘性的EPS在海洋湍流环境中会通过碰撞粘附作用促进海油雪的形成。分散剂对于海油雪的影响存在争议,一方面能够促进石油在海水中的分散,导致更多小油滴的形成,增加石油与悬浮粒子的碰撞几率,也可作为微生物的碳源和能量来源,刺激EPS的产生,促进海油雪的形成。另一方面,分散剂会通过影响微生物分泌EPS的能力、分散已经形成的凝胶、改变海水中的微生物群落等途径抑制海油雪的形成,这可能与微生物群落、石油类型、海洋环境条件等因素有关,有待进一步澄清。海油雪为微生物群落的存在提供了基质,促进了其中石油组分的降解,影响了石油组分的风化。沉降到海底的海油雪通过摄食、微生物活动、窒息、生物毒性等途径对底栖生物产生影响,同时也会影响污染物汞的迁移转化,对生态系统产生影响。
关于海油雪的相关研究近几年才系统开展,主要集中在形成机制和底栖生物毒性方面。人们对海洋石油烃降解菌群落的演替和降解路径进行了充分的分析,但是以海油雪为介质的研究则较少,未来工作应该结合分子表征和基因表达技术为海油雪中石油烃的微生物降解提供直接证据。
References
| [1] | Fu J, Gong YY, Zhao X, O'Reilly SE, Zhao DY. Effects of oil and dispersant on formation of marine oil snow and transport of oil hydrocarbons. Environmental Science & Technology, 2014, 48(24): 14392-14399. |
| [2] | Alldredge AL, Silver MW. Characteristics, dynamics and significance of marine snow. Progress in Oceanography, 1988, 20(1): 41-82. |
| [3] | Thornton DCO. Diatom aggregation in the sea:mechanisms and ecological implications. European Journal of Phycology, 2002, 37(2): 149-161. |
| [4] | Silver MW, Shanks AL, Trent JD. Marine snow:microplankton habitat and source of small-scale patchiness in pelagic populations. Science, 1978, 201(4353): 371-373. |
| [5] | Turner JT. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Progress in Oceanography, 2015, 130: 205-248. |
| [6] | Xu C, Lin P, Zhang SJ, Sun LN, Xing W, Schwehr KA, Chin WC, Wade TL, Knap AH, Hatcher PG, Yard A, Jiang C, Quigg A, Santschi PH. The interplay of extracellular polymeric substances and oil/Corexit to affect the petroleum incorporation into sinking marine oil snow in four mesocosms. Science of the Total Environment, 2019, 693: 133626. |
| [7] | Passow U, Ziervogel K, Asper V, Diercks A. Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environmental Research Letters, 2012, 7(3): 035301. |
| [8] | Tooby PF, Wick GL, Isaacs JD. The motion of a small sphere in a rotating velocity field:a possible mechanism for suspending particles in turbulence. Journal of Geophysical Research, 1977, 82(15): 2096-2100. |
| [9] | Logan BE. Environmental transport processes. 2nd ed. New York: John Wiley & Sons, 2012. |
| [10] | Ki?rboe T. Small-scale turbulence, marine snow formation, and planktivorous feeding. Scientia Marina, 1997, 61(S1): 141-158. |
| [11] | Jackson GA. A model of the formation of marine algal flocs by physical coagulation processes. Deep Sea Research Part A.. Oceanographic Research Papers, 1990, 37(8): 1197-1211. |
| [12] | Ki?rboe T, Andersen KP, Dam HG. Coagulation efficiency and aggregate formation in marine phytoplankton. Marine Biology, 1990, 107(2): 235-245. |
| [13] | van Eenennaam JS, Wei YZ, Grolle KCF, Foekema EM, Murk AJ. Oil spill dispersants induce formation of marine snow by phytoplankton-associated bacteria. Marine Pollution Bulletin, 2016, 104(1/2): 294-302. |
| [14] | Gutierrez T, Berry D, Yang TT, Mishamandani S, McKay L, Teske A, Aitken MD. Role of bacterial exopolysaccharides (EPS) in the fate of the oil released during the Deepwater Horizon Oil Spill. PLoS One, 2013, 8(6): e67717. |
| [15] | Metzger U, Lankes U, Fischpera K, Frimmel FH. The concentration of polysaccharides and proteins in EPS of Pseudomonas putida and Aureobasidum pullulans as revealed by 13C CPMAS NMR spectroscopy. Applied Microbiology and Biotechnology, 2009, 85(1): 197-206. |
| [16] | Corzo A, Morillo JA, Rodríguez S. Production of transparent exopolymer particles (TEP) in cultures of Chaetoceros calcitrans under nitrogen limitation. Aquatic Microbial Ecology, 2000, 23(1): 63-72. |
| [17] | de Jesus Raposo MF, de Morais RMSC, de Morais AMMB. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Marine Drugs, 2013, 11(1): 233-252. |
| [18] | Arnosti C, Ziervogel K, Yang TT, Teske A. Oil-derived marine aggregates-hot spots of polysaccharide degradation by specialized bacterial communities. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2016, 129: 179-186. |
| [19] | Leppard GG. Colloidal organic fibrils of acid polysaccharides in surface waters:electron-optical characteristics, activities and chemical estimates of abundance. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 1997, 120(1/3): 1-15. |
| [20] | Chin WC, Orellana MV, Verdugo P. Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature, 1998, 391(6667): 568-572. |
| [21] | Verdugo P, Santschi PH. Polymer dynamics of DOC networks and gel formation in seawater. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2010, 57(16): 1486-1493. |
| [22] | Passow U, Alldredge AL, Logan BE. The role of particulate carbohydrate exudates in the flocculation of diatom blooms. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 1994, 41(2): 335-357. |
| [23] | Yu GH, He PJ, Shao LM. Characteristics of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability. Bioresource Technology, 2009, 100(13): 3193-3198. |
| [24] | Shanks AL, Edmondson EW. Laboratory-made artificial marine snow:a biological model of the real thing. Marine Biology, 1989, 101(4): 463-470. |
| [25] | Suja LD, Summers S, Gutierrez T. Role of EPS, Dispersant and nutrients on the microbial response and MOS formation in the Subarctic Northeast Atlantic. Frontiers in Microbiology, 2017, 8: 676. |
| [26] | Passow U. Formation of rapidly-sinking, oil-associated marine snow. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2016, 129: 232-240. |
| [27] | Valentine DL, Kessler JD, Redmond MC, Mendes SD, Heintz MB, Farwell C, Hu L, Kinnaman FS, Yvon-Lewis S, Du MR, Chan EW, Tigreros FG, Villanueva CJ. Propane respiration jump-starts microbial response to a deep oil spill. Science, 2010, 330(6001): 208-211. |
| [28] | McGenity TJ. Hydrocarbon biodegradation in intertidal wetland sediments. Current Opinion in Biotechnology, 2014, 27: 46-54. |
| [29] | Quigg A, Passow U, Chin WC, Xu C, Doyle S, Bretherton L, Kamalanathan M, Williams AK, Sylvan JB, Finkel ZV, Knap AH, Schwehr KA, Zhang SJ, Sun LN, Wade TL, Obeid W, Hatcher PG, Santschi PH. The role of microbial exopolymers in determining the fate of oil and chemical dispersants in the ocean. Limnology and Oceanography Letters, 2016, 1(1): 3-26. |
| [30] | Gong YY, Zhao X, O'Reilly SE, Qian TW, Zhao DY. Effects of oil dispersant and oil on sorption and desorption of phenanthrene with Gulf Coast marine sediments. Environmental Pollution, 2014, 185: 240-249. |
| [31] | Thibodeaux LJ, Valsaraj KT, John VT, Papadopoulos KD, Pratt LR, Pesika NS. Marine oil fate:knowledge gaps, basic research, and development needs; a perspective based on the deepwater horizon spill. Environmental Engineering Science, 2011, 28(2): 87-93. |
| [32] | Ramachandran SD, Hodson PV, Khan CW, Lee K. Oil dispersant increases PAH uptake by fish exposed to crude oil. Ecotoxicology And Environmental Safety, 2004, 59(3): 300-308. |
| [33] | Cai ZQ, Gong YY, Liu W, Fu J, O'Reilly SE, Hao XD, Zhao DY. A surface tension based method for measuring oil dispersant concentration in seawater. Marine Pollution Bulletin, 2016, 109(1): 49-54. |
| [34] | Kujawinski EB, Soule MCK, Valentine DL, Boysen AK, Longnecker K, Redmond MC. Fate of dispersants associated with the deepwater horizon oil spill. Environmental Science & Technology, 2011, 45(4): 1298-1306. |
| [35] | Zhao X, Liu W, Fu J, Cai ZQ, O'Reilly SE, Zhao DY. Dispersion, sorption and photodegradation of petroleum hydrocarbons in dispersant-seawater-sediment systems. Marine Pollution Bulletin, 2016, 109(1): 526-538. |
| [36] | Hemmer MJ, Barron MG, Greene RM. Comparative toxicity of eight oil dispersants, Louisiana sweet crude oil (LSC), and chemically dispersed LSC to two aquatic test species. Environmental Toxicology and Chemistry, 2011, 30(10): 2244-2252. |
| [37] | Fu J, Gong YY, Cai ZQ, O'Reilly SE, Zhao DY. Mechanistic investigation into sunlight-facilitated photodegradation of pyrene in seawater with oil dispersants. Marine Pollution Bulletin, 2017, 114(2): 751-758. |
| [38] | 刘迪.分散剂作用下溢油乳化和生物降解效果研究.青岛理工大学硕士学位论文, 2013. |
| [39] | Li ZK, Kepkay P, Lee K, King T, Boufadel MC, Venosa AD. Effects of chemical dispersants and mineral fines on crude oil dispersion in a wave tank under breaking waves. Marine Pollution Bulletin, 2007, 54(7): 983-993. |
| [40] | Suja LD, Chen XD, Summers S, Paterson DM, Gutierrez T. Chemical dispersant enhances microbial exopolymer (EPS) production and formation of marine oil/dispersant snow in surface waters of the Subarctic Northeast Atlantic. Frontiers in Microbiology, 2019, 10: 553. |
| [41] | 韩璐.分散剂的投加对海洋石油雪形成及沉降特性的影响.青岛理工大学硕士学位论文, 2018. |
| [42] | Chiu MH, Vazquez CI, Shiu RF, Le C, Sanchez NR, Kagiri A, Garcia CA, Nguyen CH, Tsai SM, Zhang SJ, Xu C, Santschi PH, Quigg A, Chin WC. Impact of exposure of crude oil and dispersant (Corexit) on aggregation of extracellular polymeric substances. Science of the Total Environment, 2019, 657: 1535-1542. |
| [43] | Kleindienst S, Seidel M, Ziervogel K, Grim S, Loftis K, Harrison S, Malkin SY, Perkins MJ, Field J, Sogin ML, Dittmar T, Passow U, Medeiros PM, Joye SB. Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(48): 14900-14905. |
| [44] | 于洋.分散剂及生物柴油对石油降解微生物多样性的影响研究.青岛理工大学硕士学位论文, 2014. |
| [45] | 李会琳.生物与化学分散剂对海洋石油降解微生物的影响.西华师范大学硕士学位论文, 2019. |
| [46] | Hamdan LJ, Fulmer PA. Effects of COREXIT? EC9500A on bacteria from a beach oiled by the Deepwater Horizon spill. Aquatic Microbial Ecology, 2011, 63(2): 101-109. |
| [47] | Baelum J, Borglin S, Chakraborty R, Fortney JL, Lamendella R, Mason OU, Auer M, Zemla M, Bill M, Conrad ME, Malfatti SA, Tringe SG, Holman HY, Hazen TC, Jansson JK. Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environmental Microbiology, 2012, 14(9): 2405-2416. |
| [48] | Gong YY, Fu J, O'Reilly SE, Zhao DY. Effects of oil dispersants on photodegradation of pyrene in marine water. Journal of Hazardous Materials, 2015, 287: 142-150. |
| [49] | Plata DL, Sharpless CM, Reddy CM. Photochemical degradation of polycyclic aromatic hydrocarbons in oil films. Environmental Science & Technology, 2008, 42(7): 2432-2438. |
| [50] | van Eenennaam JS, Rahsepar S, Radovic JR, Oldenburg TBP, Wonink J, Langenhoff AAM, Murk AJ, Foekema EM. Marine snow increases the adverse effects of oil on benthic invertebrates. Marine Pollution Bulletin, 2018, 126: 339-348. |
| [51] | Passow U, Ziervogel K. Marine snow sedimented oil released during the deepwater horizon spill. Oceanography, 2016, 29(3): 118-125. |
| [52] | Ziervogel K, McKay L, Rhodes B, Osburn CL, Dickson-Brown J, Arnosti C, Teske A. Microbial activities and dissolved organic matter dynamics in oil-contaminated surface seawater from the Deepwater Horizon Oil Spill site. PLoS One, 2012, 7(4): e34816. |
| [53] | Wirth MA, Passow U, Jeschek J, Hand I, Schulz-Bull DE. Partitioning of oil compounds into marine oil snow:insights into prevailing mechanisms and dispersant effects. Marine Chemistry, 2018, 206: 62-73. |
| [54] | Wozniak AS, Prem PM, Obeid W, Waggoner DC, Quigg A, Xu C, Santschi PH, Schwehr KA, Hatcher PG. Rapid degradation of oil in mesocosm simulations of marine oil snow events. Environmental Science & Technology, 2019, 53(7): 3441-3450. |
| [55] | Duran R, Cravo-Laureau C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiology Reviews, 2016, 40(6): 814-830. |
| [56] | Rodriguez-R LM, Overholt WA, Hagan C, Huettel M, Kostka JE, Konstantinidis KT. Microbial community successional patterns in beach sands impacted by the Deepwater Horizon oil spill. The ISME Journal, 2015, 9(9): 1928-1940. |
| [57] | Mason OU, Scott NM, Gonzalez A, Robbins-Pianka A, B?lum J, Kimbrel J, Bouskill NJ, Prestat E, Borglin S, Joyner DC, Fortney JL, Jurelevicius D, Stringfellow WT, Alvarez-Cohen L, Hazen TC, Knight R, Gilbert JA, Jansson JK. Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. The ISME Journal, 2014, 8(7): 1464-1475. |
| [58] | Daly KL, Passow U, Chanton J, Hollande D. Assessing the impacts of oil-associated marine snow formation and sedimentation during and after the Deepwater Horizon oil spill. Anthropocene, 2016, 13: 18-33. |
| [59] | Du YF, Gao S, Liu XS, Wang DD, Zhang LH, Ingels J. Meiofauna and nematode community characteristics indicate ecological changes induced by geomorphic evolution:a case study on tidal creek systems. Ecological Indicators, 2018, 87: 97-106. |
| [60] | Shiells GM, Anderson KJ. Pollution monitoring using the nematode/copepod ratio A practical application. Marine Pollution Bulletin, 1985, 16(2): 62-68. |
| [61] | Rohal M, Barrera N, Van Eenennaam JS, Foekema EM, Montagna PA, Murk AJ, Pryor M, Romero IC. The effects of experimental oil-contaminated marine snow on meiofauna in a microcosm. Marine Pollution Bulletin, 2020, 150: 110656. |
| [62] | Kang T, Oh JH, Hong JS, Kim D. Responses of meiofauna and nematode communities to crude oil contamination in a laboratory microcosm experiment. Ocean Science Journal, 2016, 51(3): 465-476. |
| [63] | Hastings DW, Schwing PT, Brooks GR, Larson RA, Morford JL, Roeder T, Quinn KA, Bartlett T, Romero IC, Hollander DJ. Changes in sediment redox conditions following the BP DWH blowout event. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2016, 129: 167-178. |
| [64] | Rahsepar S, Langenhoff AAM, Smit MPJ, Van Eenennaam JS, Murk AJ, Rijnaarts HHM. Oil biodegradation:interactions of artificial marine snow, clay particles, oil and Corexit. Marine Pollution Bulletin, 2017, 125(1/2): 186-191. |
| [65] | Schwing PT, Romero IC, Brooks GR, Hastings DW, Larson RA, Hollander DJ. A decline in benthic foraminifera following the Deepwater Horizon Event in the Northeastern Gulf of Mexico. PLoS One, 2015, 10(3): e0120565. |
| [66] | Liu Y, Shi Q, Zhang YH, He YL, Chung KH, Zhao SQ, Xu CM. Characterization of red pine pyrolysis bio-oil by gas chromatography-mass spectrometry and negative-ion electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Energy & Fuels, 2012, 26(7): 4532-4539. |
| [67] | Fisher CR, Hsing PY, Kaiser CL, Yoerger DR, Roberts HH, Shedd WW, Cordes EE, Shank TM, Berlet SP, Saunders MG, Larcom EA, Brooks JM. Footprint of Deepwater horizon blowout impact to deep-water coral communities. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(32): 11744-11749. |
| [68] | DeLeo DM, Ruiz-Ramos DV, Baums IB, Cordes EE. Response of deep-water corals to oil and chemical dispersant exposure. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2016, 129: 137-147. |
| [69] | Murawski SA, Hogarth WT, Peebles EB, Barbeiri L. Prevalence of external skin lesions and polycyclic aromatic hydrocarbon concentrations in Gulf of Mexico fishes, post-Deepwater Horizon. Transactions of the American Fisheries Society, 2014, 143(4): 1084-1097. |
| [70] | Snyder SM, Pulster EL, Wetzel DL, Murawski SA. PAH exposure in Gulf of Mexico demersal fishes, post-Deepwater horizon. Environmental Science & Technology, 2015, 49(14): 8786-8795. |
| [71] | Lampitt RS, Wishner KF, Turley CM, Angel MV. Marine snow studies in the Northeast Atlantic Ocean:distribution, composition and role as a food source for migrating plankton. Marine Biology, 1993, 116(4): 689-702. |
| [72] | Ziervogel K, Joye SB, Arnosti C. Microbial enzymatic activity and secondary production in sediments affected by the sedimentation pulse following the Deepwater Horizon oil spill. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2016, 129: 241-248. |
| [73] | Lasorsa BK, Gill GA, Horvat M. Analytical methods for measuring mercury in water, sediment and biota. Richland, WA: Pacific Northwest National Lab., 2012: 27-54. |
| [74] | Parks JM, Johs A, Podar M, Bridou R, Hurt Jr RA, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC, Palumbo AV, Smith JC, Wall JD, Elias DA, Liang LY. The genetic basis for bacterial mercury methylation. Science, 2013, 339(6125): 1332-1335. |
| [75] | Bridou R, Monperrus M, Gonzalez PR, Guyoneaud R, Amouroux D. Simultaneous determination of mercury methylation and demethylation capacities of various sulfate-reducing bacteria using species-specific isotopic tracers. Environmental Toxicology and Chemistry, 2011, 30(2): 337-344. |
| [76] | Fujimura M, Usuki F. In situ different antioxidative systems contribute to the site-specific methylmercury neurotoxicity in mice. Toxicology, 2017, 392: 55-63. |
| [77] | Joye SB, Teske AP, Kostka JE. Microbial dynamics following the Macondo oil well blowout across Gulf of Mexico environments. Bioscience, 2014, 64(9): 766-777. |
| [78] | Kleindienst S, Paul JH, Joye SB. Using dispersants after oil spills:impacts on the composition and activity of microbial communities. Nature Reviews Microbiology, 2015, 13(6): 388-396. |
| [79] | Kim S, Stanford LA, Rodgers RP, Marshall AG, Walters CC, Qian KN, Wenger LM, Mankiewicz P. Microbial alteration of the acidic and neutral polar NSO compounds revealed by Fourier transform ion cyclotron resonance mass spectrometry. Organic Geochemistry, 2005, 36(8): 1117-1134. |
| [80] | Zhu W, Song Y, Adediran GA, Jiang T, Reis AT, Pereira E, Skyllberg U, Bj?rn E. Mercury transformations in resuspended contaminated sediment controlled by redox conditions, chemical speciation and sources of organic matter. Geochimica et Cosmochimica Acta, 2018, 220: 158-179. |
| [81] | Perrot V, Landing WM, Grubbs RD, Salters VJM. Mercury bioaccumulation in tilefish from the northeastern Gulf of Mexico 2 years after the Deepwater Horizon oil spill:insights from Hg, C, N and S stable isotopes. Science of the Total Environment, 2019, 666: 828-838. |
| [82] | Wallschl?ger D, Desai MVM, Wilken RD. The role of humic substances in the aqueous mobilization of mercury from contaminated floodplain soils. Water, Air, and Soil Pollution, 1996, 90(3/4): 507-520. |
| [83] | Driscoll CT, Blette V, Yan C, Schofield CL, Munson R, Holsapple J. The role of dissolved organic carbon in the chemistry and bioavailability of mercury in Remote Adirondack Lakes. Water, Air, and Soil Pollution, 1995, 80(1/4): 499-508. |
| [84] | Ainsworth CH, Paris CB, Perlin N, Dornberger LN, Patterson III WF, Chancellor E, Murawski S, Hollander D, Daly K, Romero IC, Coleman F, Perryman H. Impacts of the Deepwater Horizon oil spill evaluated using an end-to-end ecosystem model. PLoS One, 2018, 13(1): e0190840. |
