孙小溪, 蒋宏忱


中国地质大学(武汉), 生物地质与环境地质国家重点实验室, 湖北 武汉 430074
收稿日期:2019-06-04;修回日期:2019-07-27;网络出版日期:2019-08-15
基金项目:国家自然科学基金(91751206);中央高校基本科研业务费专项
作者简介:蒋宏忱, 博士, 中国地质大学(武汉)生物地质与环境地质国家重点实验室教授、博导, 2007年获美国迈阿密大学博士学位。2012年入选教育部"新世纪优秀人才支持计划", 2014年获国家优秀青年基金。现任中国微生物学会地质微生物专业委员会委员、中国古生物学会地球生物学分会理事、Frontiers in Microbiology、《盐湖研究》与《微生物学报》编委。致力于盐湖和热泉等极端环境地质微生物学研究。先后主持了国家基金委重大研究计划重点项目、国家优秀青年基金项目等重要课题, 已在Environmental Microbiology、Applied and Environmental Microbiology等专业期刊发表科研论文100余篇。2015年获中国地质学会青年地质科技奖-银锤奖, 2017年获云南省自然科学二等奖(排名第二).
*通信作者:蒋宏忱。Tel:+86-27-67883452;Fax:+86-27-67883451;E-mail:jiangh@cug.edu.cn.
摘要:湖泊中微生物介导的反硝化过程对于区域乃至全球的气候环境变化有着深远的影响。因此,研究湖泊微生物反硝化过程及速率有助于我们深刻理解湖泊氮元素生物地球化学循环规律,全面认识湖泊生境对全球氮循环的贡献。本文综述了湖泊生境中反硝化过程(包括典型的反硝化过程及与其他物质循环耦合的反硝化过程,如与有机氮耦合的共反硝化作用、与碳循环耦合的硝酸盐/亚硝酸盐依赖型厌氧甲烷氧化、与铁循环耦合的硝酸盐依赖型铁氧化、与硫循环耦合的硝酸盐还原硫氧化)的速率、驱动微生物及其影响因素。最后对湖泊反硝化过程研究现状和未来发展方向提出总结与展望。
关键词:湖泊微生物反硝化速率影响因素
Progress in microbially mediated denitrifying in lacustrine environments
Xiaoxi Sun, Hongchen Jiang


State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences(Wuhan), Wuhan 430074, Hubei Province, China
Received: 4 June 2019; Revised: 27 July 2019; Published online: 15 August 2019
*Corresponding author: Hongchen Jiang, Tel: +86-27-67883452; Fax: +86-27-67883451; E-mail: jiangh@cug.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (91751206) and by the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan)
Abstract: Microbially mediated denitrification in lacustrine ecosystems has profound impact on regional and even global environment and climate change. Thus, studying lake microbial denitrification process and rate helps us obtain comprehensive understanding of nitrogen biogeochemical cycle in lacustrine ecosystems and its role in the global nitrogen cycle. This review summarizes the denitrification processes, rates and involved microbial community compositions in lacustrine ecosystems and their influencing factors. The covered denitrification processes include typical denitrification processes and those coupled with other elemental cycles including co-denitrification coupled with organic nitrogen, nitrate/nitrite-dependent anaerobic oxidization of methane coupled with carbon cycle, anaerobic nitrate-dependent Fe(Ⅱ) oxidation coupled with iron cycle, nitrate/nitrite-dependent sulfide oxidation coupled with sulfur cycle. Finally, current researches and future directions related to denitrification processes in lacustrine environments were summarized.
Keywords: lakesmicrobesdenitrificationrateinfluencing factors
内陆水生系统在全球氮循环中扮演着重要的角色。其面积不足陆地面积的4%,却贡献了约50%的陆地氮流失[1]。其每年运移、矿化、埋藏的氮总量(0.1 Pg,Pg=1015 g)约等于人类工业活动导致的陆地氮输入(0.1 Pg),接近海洋总固氮量(0.14 Pg)[2] (表 1)。因此,研究内陆水生系统的氮循环过程能够帮助我们科学、全面地认识全球氮循环。
表 1. 全球氮通量 Table 1. Global nitrogen fluxes
| Pathways | Nitrogen fluxes (Tg N/yr) | Ranges | References |
| Sources | 483 | ||
| Biological nitrogen fixation of inland waters | 74 | \ | [3] |
| Nitrogen deposition of inland waters | 23 | \ | [4] |
| Terrestrial nitrogen input of inland waters | 7 | \ | [4] |
| Terrestrial nitrogen fixation | 84 | 40–127 | [5-6] |
| Marine nitrogen fixation | 134 | 94–175 | [7-8] |
| Lighting | 5 | \ | [5] |
| Industrial nitrogen fixation | 105 | 100–110 | [5, 9] |
| NOx from combustion | 38 | 25–52 | [5] |
| Agricultural nitrogen fixation | 43 | 32–50 | [5, 10] |
| Sinks | 521 | ||
| Inland waters denitrification | 110 | 39–216 | [1] |
| Inland waters N2O emission | 0.22 | 0.15–0.28 | [11] |
| Terrestrial denitrification | 130 | 58–175 | [1] |
| Marine denitrification | 245 | 107–331 | [5, 7] |
| Terrestrial N2O emission | 12 | \ | [2] |
| Marine N2O emission | 4 | \ | [2] |
| Marine nitrogen burial | 20 | 16–25 | [2, 5, 12] |
| “\” means no reference data. | |||
表选项
湖泊作为内陆水体的主要组成部分,约占其总面积的83%,且湖泊单位面积的氮去除速率与河流相当,远高于其他内陆水体(如地下水),在内陆水体氮循环中扮演着举足轻重的角色[1]。微生物作为湖泊氮循环的主要驱动者,参与推动湖泊氮固定、运移、循环转化以及埋藏等多个重要的氮循环过程。因此,研究湖泊微生物驱动的氮循环过程有助于我们深刻理解湖泊氮循环的机制,全面认识湖泊氮循环在全球生物化学循环中的作用。
由于承受着陆源过量的营养盐输入与持续增长的NO3–型氮沉降,湖泊生境生态功能有着明显的退化趋势[13-14]。反硝化过程在减轻湖泊氮载荷方面起着不可替代的作用,能够将湖泊中的硝态氮转化为N2O或N2。在某些湖泊中,氮的去除率高达36% (与氮输入总量相比)[15]。然而,N2O是一种强大温室气体,其增温潜势是CO2的约300倍,还会在平流层进一步氧化为NO破坏臭氧层,因此,N2O排放速率也越来越受到人们的广泛关注[16-17]。除了典型的反硝化过程,近年来与有机氮、甲烷、铁、硫等元素或化合物耦合的反硝化新途径也开始进入****们的视野(图 1),这些新途径对于湖泊的氮去除过程也有一定程度的贡献[18-21]。本文将综述湖泊生境中不同类型的反硝化过程,介绍其速率及驱动微生物的群落组成,并综合微生物代谢机理分析反硝化过程的影响因素,总结现有研究的不足,展望未来研究的方向。
 |
| 图 1 反硝化与其他元素循环耦合模式图 Figure 1 Typical denitrification processes and those coupled with other elemental cycles. N-DAMO: Nitrite-Dependent Anaerobic Methane-Oxidization; ANDFO: Anaerobic Nitrate-Dependent Fe(Ⅱ) Oxidation; NAR: nitrate reductase; NIR: nitrite reductase; NOR: nitric oxide reductase; NOS: nitric oxide synthase; E-NO complex: enzyme (E) bound NO complexes; NOD: nitric oxide dismutase. |
| 图选项 |
1 湖泊中的反硝化过程 1.1 硝酸盐还原为亚硝酸盐 反硝化作用中的硝酸盐还原指的是微生物通过呼吸作用将硝酸盐转化为亚硝酸盐的异化还原过程。硝酸盐的异化还原过程主要依靠兼性厌氧的细菌、古菌和真核生物(如α、β和γ变形菌纲的细菌和一些嗜盐古菌)等完成[22-23]。相对于其他氮循环过程,硝酸盐还原是湖泊中亚硝酸盐的主要贡献者。而亚硝酸盐作为中间产物继而通过三种途径被消耗[22, 24-25]:(1)通过反硝化作用被还原为气态产物(N2O或N2);(2)通过硝酸盐异化作用被还原成铵(DNRA,又称硝酸盐铵化);(3)通过厌氧氨氧化(anammox)过程被还原为氮气。
1.2 亚硝酸盐还原为一氧化氮 湖泊反硝化作用中的亚硝酸盐还原过程主要由变形菌和拟杆菌完成[26-28]。亚硝酸盐还原为一氧化氮的反应可以由两种结构不同但功能相似的酶来实现:含血红素的cd1亚硝酸盐还原酶(cd1-NIR)和含铜的亚硝酸盐还原酶(Cu-NIR)。这两种酶分别由nirS和nirK基因编码,其中nirS基因的分布更广,存在于接近75%的反硝化微生物中[22, 29]。这两种酶都位于细胞周质,不能贡献能量保存[23, 28]。它们的区别在于催化亚硝酸盐还原产物的差异:cd1-NIR的反应产物往往是NO,而Cu-NIR在强还原和高pH条件下可能催化形成N2O[22]。
1.3 一氧化氮还原为氧化亚氮 一氧化氮作为反硝化的中间产物,具有细胞毒性,几乎不在胞内积累,很快在细胞内被还原或被微生物排泄到环境中[22, 30]。一氧化氮还原为氧化亚氮的过程是由一氧化氮还原酶(NOR)催化实现的,由norB或norZ基因编码[31-33]。
N2O是一种强烈的温室气体,其释放速率与湖泊氮输入密切相关。McCrackin统计了26个不同类型(不同营养状态)湖泊的相关数据发现,N2O释放速率与湖泊硝态氮载荷呈显著正相关[34]。本文在此基础上统计了全球180个不同营养状态湖泊的N2O释放数据(涵盖了寡营养、中营养、富营养、重度富营养湖泊),发现N2O释放速率有随湖泊富营养化逐渐升高的趋势(表 2)。据估计,全球湖泊每年N2O排放量为0.04–2.00 Tg N,约占内陆水体总排放量的12%–49%,到2050年N2O排放量将增长至0.1–3.4 Tg N/yr[34-35]。
表 2. 不同营养类型湖泊的N2O释放速率 Table 2. N2O emission rates in lakes of different nutrient types
| Lakes | Descriptions | N2O flux/[μmol N/(m2·d)] | References | ||
| System mean | Lake mean | Range | |||
| 121 small oligotrophic lakes | Oligotrophic | 2.3 | 2.6 | –7.6–13.8 | [36] |
| Lake Makijarvi | Oligotrophic | 2.3 | –0.48–4.2 | [37] | |
| 25 low-deposition region lakes | Oligotrophic | 1.2 | 0–24 | [34, 38] | |
| Jankalaisenlampi pond | Mesotrophic | 14.8 | 0.68 | [37] | |
| Kotsamolampi pond | Mesotrophic | –0.28 | [37] | ||
| Lake Huahu | Mesotrophic | 44 | –38–191 | [39] | |
| 24 high-deposition region lakes | Eutrophic | 18.8 | 22.4 | –24–163.2 | [34, 38] |
| Lake Okaro | Eutrophic | 1.2 | [40] | ||
| Lake Postilampi | Eutrophic | 4.9 | –0.22–14 | [37] | |
| Lake Heinalampi | Eutrophic | –3.6 | [37] | ||
| Lake Kevaton | Eutrophic | –2.16 | –1.12– –0.32 | [37] | |
| Lake Vehmasjarvi | Eutrophic | 7 | –0.26–11.8 | [37] | |
| Taihu Lake | Hyper-eutrophic | 23.7 | 23.7 | –3.4–109.8 | [41] |
| Negative flux rates indicate movement of N2O from the atmosphere to the lake. | |||||
表选项
湖泊N2O释放速率具有很强的时空异质性,主要受到盐度、pH、DO、C/N比、NO3–浓度等环境因子的影响,具有季节性变化的特征。对反硝化微生物而言,非最适的生存条件(如高浓度氧气和低C/N比)往往会刺激其产生N2O[42]。盐度的升高和pH的降低也会刺激N2O释放[43-44]。高浓度DO (如5%)会抑制氧化亚氮还原酶的合成与活性从而增加N2O的释放,但也不利于反硝化作用的进行,综合来看高浓度DO会导致反硝化过程中N2O/N2比增加[45]。N2O释放量往往随着C/N比升高而降低,可能与反硝化微生物的能量需求有关,在C/N比较高(即碳源充足)的情况下,异养的反硝化微生物倾向于实现NO3–到N2的完全转化[38, 46]。NO3–作为反硝化作用的底物,其浓度与湖泊N2O产量具有显著的正相关关系[34]。N2O释放表现出明显的季节性特征实质上是受到温度和降水等因素的调控,一般在夏季出现峰值[47-48]。
1.4 氧化亚氮还原为氮气 微生物将氧化亚氮还原为氮气是减少氧化亚氮这种强大温室气体的主要途径。氧化亚氮还原酶(NOS,由nosZDFYL基因编码)是目前已知的唯一能够催化此反应的酶,广泛存在于变形菌门、拟杆菌门、绿菌门以及古菌中的泉古菌门和盐杆菌纲中[49-50]。尽管能将硝酸根转化为氮气的完全反硝化菌更为常见,对于某些缺少NOS的微生物来说氧化亚氮即是反硝化的最终产物(如Pseudomonas chlororaphis和某些Rhizobium)[22]。
氧化亚氮还原为氮气的速率主要受到两方面因素的影响,即不完全反硝化菌的生态位分异(亚硝酸盐还原菌和氧化亚氮还原菌的分布不均衡导致局部氧化亚氮积累/不足)和完全反硝化菌的NOS酶活性(与其他反硝化酶相比,NOS更加敏感,其活性容易受到氧气、pH以及硫化物的影响)[50-53]。值得注意的是,青藏高原盐湖中亚硝酸盐还原菌和氧化亚氮还原菌群落组成的差异显著,受到不同环境因子的调控,其中氧化亚氮还原菌对环境变化更为敏感,暗示着盐湖生境中可能是由不同微生物在实现完全反硝化过程(未发表数据)。这种不完全反硝化菌的差异是否会导致盐湖N2O的局部积累或不足值得我们进一步探究。此外,随着纬度的增加,湖泊N2O的排放速率逐级降低,表现出从N2O源转变为N2O汇的趋势,且作为N2O汇的湖泊往往具有低pH (< 6.275)高DOC (> 7.492 mg/L)和低溶氧(< 7.805 mg/L)的特征[54]。
1.5 湖泊反硝化速率及其影响因素 反硝化过程是湖泊氮去除的重要途径,其去除的氮总量约占湖泊氮输入的1%–36%[15]。从全球范围来看,湖泊的反硝化通量为19–43 Tg/yr,约占陆地氮输入的7%–16%和全球反硝化通量的4%–9%;其中,较小的湖泊(< 50 km2)大约贡献了2/3的湖泊反硝化总量[1]。
大部分反硝化作用发生在湖泊的下层滞水带及沉积物等低氧层位(一般为氧气浓度低于0.2 mg/L的次氧化层)。不同层位的反硝化速率差异很大,例如近岸表层沉积物反硝化速率为22.5– 366.2 nmol N/(L·h),湖泊水体的反硝化速率为6.0–13.3 nmol N/(L·h)[55-59]。沉积物反硝化过程的氮源一般由沉积物本身矿化产生的硝酸根提供,而不是上覆水体中的氮,因而大量无机氮会通过反硝化作用从沉积物中去除(76%–100%)[15, 56]。
从宏观上来看,湖泊生态系统反硝化速率的影响因素主要有水动力条件和氮载荷量[1]。湖泊的水动力条件通过控制湖水中氮素的滞留时间来影响湖泊的反硝化速率。通常湖水滞留时间越长,参与反硝化过程的氮素比例越高,越有利于湖泊的反硝化作用。而湖泊氮载荷量决定了反硝化作用中底物的上限,通常湖泊的氮载荷量越高,其反硝化速率也越高[60]。
从微观上来看,湖泊反硝化速率的影响因素主要有氧气浓度(影响酶活性)、底物(硝酸根和有机质)可利用性以及温度(季节性变化和昼夜变化)和盐度[22]。本研究从文献中[56, 58-59]统计了长江中下游盆地22个富营养湖泊的反硝化速率、地化以及生物信息,使用R软件(Version 3.5.1) RandomForest (随机森林)程序包计算了不同环境因子对微生物反硝化速率的相对重要性。随机森林评价功能中的Inc MSE指数等价于Mean Decrease Accuracy,描述的是对某个环境参数随机赋值时,随机森林预测准确度的降低程度,Inc MSE越大表示环境因子的相对重要性越高(Inc MSE等于0说明该环境参数与反硝化速率在统计学上不相关,Inc MSE小于0则无统计学意义)[61]。结果显示,对湖泊反硝化速率影响最大的前三个环境因子分别是沉积物硝酸盐浓度、溶解性总固体(可理解为盐度)和溶解氧(图 2),与前人的研究结论一致[56, 58-59, 62-64]。
 |
| 图 2 环境因子对湖泊反硝化速率的相对贡献率 Figure 2 Relative contributions of environmental factors to lacustrine denitrification rates. |
| 图选项 |
由于反硝化作用是一种高产能的异化还原过程,伴随着大量ATP的产生,因此能够耐受盐度胁迫,可在超盐环境(如盐湖,甚至盐度接近饱和的超盐湖)中发生[63, 65-66]。值得注意的是,高盐环境中反硝化微生物的生长似乎没有受到盐度的影响,但能够观察到反硝化终产物从氮气向氧化亚氮的转变[65]。结合NOS比其他反硝化酶更加敏感的特征,该酶是否更容易受到盐度的抑制作用,使得盐湖有着更高的氧化亚氮释放速率,值得我们进一步探究。因此,全面评估盐湖的反硝化速率及其反硝化产物(氮气与氧化亚氮)比例对于全面评估全球氮循环具有重要意义。
2 与其他代谢过程耦合的反硝化新途径 2.1 共反硝化作用 早在19世纪末,****们就发现微生物介导的硝酸盐还原过程中会产生额外的氮气,额外产生氮气的来源一直困扰着研究人员。直到20世纪末,同位素标定技术的使用和共反硝化作用(codenitrification)的发现才回答了这个问题。共反硝化作用会产生杂交氧化亚氮和氮气[18],这种与传统反硝化作用共代谢的新途径通过微生物介导的亚硝化反应形成N-N键,即亚硝酸盐或一氧化氮与其他含氮化合物(胺类、羟胺、铵根、叠氮化物、联氨等)各贡献一个氮原子生成氧化亚氮、氮气或同时生成两种气体(图 3)[18, 67-69]。据现有研究,共反硝化作用存在于细菌、古菌和真菌三域中,但仅有为数不多的微生物能够执行该反应[70–72]。共反硝化作用已被证实在土壤环境中扮演着重要的角色(如草地中92%的氮气由共反硝化作用产生[73]),而在水体环境中,共反硝化过程的分布和速率仍有待进一步研究。前人研究显示,湖水中氧化亚氮分子内氮同位素存在不一致的异常现象,这暗示了水体环境中可能存在共反硝化作用[74-75]。此外,水体中浓度合适(μmol/L级别)的氨基化合物也能够为共反硝化作用提供潜在底物[76-77]。
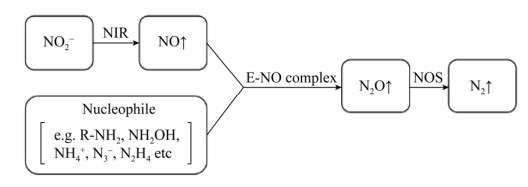 |
| 图 3 共反硝化作用模式图(修改自参考文献[68]) Figure 3 Schematic diagram of codenitrification (modified after reference [68]). |
| 图选项 |
基于共代谢的特征,影响反硝化过程的环境因子对共反硝化作用也有着相似的影响,包括氧气和有机碳底物的可利用性、pH等[68]。此外,电子供体的类型很可能主导着共反硝化作用产物的种类(杂交氮气、杂交氧化亚氮或两种气体同时产生):当电子供体为–3价(如R-NH2、NH3)时会生成氮气;当电子供体为–1价(如NH2OH)时会生成氧化亚氮;当电子供体为–2价(如联氨)时会同时生成氧化亚氮和氮气[18]。
2.2 厌氧甲烷氧化耦合反硝化 微生物介导的碳、氮元素循环过程通常耦合在一起,并在很大程度上影响着湖泊的初级生产力、营养水平、温室气体释放等生态过程。近年来,研究人员发现一种包含细菌和古菌的富集培养物能够将厌氧甲烷氧化过程与反硝化过程耦合在一起,其中古菌Candidatus Methanoperedens nitroreducens属于厌氧甲烷氧化ANME-2d进化枝,能够在完成厌氧甲烷氧化过程的同时,将硝酸盐还原为亚硝酸盐;细菌Candidatus Methylomirabilis oxyfera属于NC10门,能够先将亚硝酸盐还原为NO,然后将NO歧化反应生成氮气和氧气,再利用胞内的氧气氧化甲烷生成二氧化碳并释放能量,该过程被称为亚硝酸盐依赖型厌氧甲烷氧化(Nitrite-dependent anaerobic methane-oxidization,N-DAMO),古菌和细菌的反应式[19, 78-80](反应式1–2)。
 | 公式(1) |
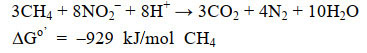 | 公式(2) |
表 3. 水生生态系统N-DAMO速率范围及甲烷氧化贡献率 Table 3. N-DAMO rate ranges and their methane oxidation contribution to aquatic ecosystems
| Ecosystems | Descriptions | Rates | Contributions/% | References |
| Lake constance | Oligotrophic | 1.8–3.6 nmol CO2/(mL·d) | 5 | [82] |
| Freshwater pond | Nitrate levels of 1–2 mmol/L | 2.3 mmol/(m2·d) | 47 | [83] |
| Xiazhuhu wetland | Eutrophic | 0.2–14.5 nmol CO2/g dry soil·d | 2.7–4.3 | [84] |
| Mangrove wetland | Salinity ~8g/L | 25.93–704.80 nmol CO2/g dry soil·d | \ | [85] |
| “\” means no reference data. | ||||
表选项
影响N-AOM过程的环境因素包括温度、氧气、硝酸根、亚硝酸根、甲烷、有机碳、盐度等。N-AOM微生物生长缓慢,倍增时间约为1–2周,因此更加倾向存在于湖泊中相对稳定的层位[86]。大量研究表明,硝酸根、亚硝酸根、甲烷等底物浓度及其配比控制着N-AOM微生物在湖泊生境中的分布和活力,例如有机碳浓度能够通过控制产甲烷菌的活力对N-AOM过程产生实质性的影响[83-84, 87-89]。盐度则是N-AOM微生物群落结构和速率的重要限制因素,前人统计了全球不同生境中N-AOM细菌的分布,发现盐度是N-AOM细菌群落组成的主控因素之一[90]。值得注意的是,前人研究显示N-AOM细菌能够存在于河口、盐湖等高盐生境(盐度高达84 g/L)中并完成反应,这暗示着嗜盐N-AOM细菌的存在[88, 91-92];而N-AOM古菌还没有在盐度超过20 g/L的环境中被报道过[93]。因此能够耐受更高盐度的N-AOM古菌是否存在仍有待进一步研究。
2.3 硝酸盐依赖型铁氧化 硝酸盐依赖型铁氧化(anaerobic nitrate- dependent Fe(Ⅱ) oxidation,ANDFO)主要发生在无光照、缺氧的中性沉积环境中,是一种微生物介导的、硝酸盐还原耦合溶解态或非溶解态铁氧化的反应,在铁、氮循环中起着不可忽视的作用(反应式3)[20, 94-96]。
 | 公式(3) |
湖泊中微生物介导的硝酸盐依赖型铁氧化过程具有重要的生态功能。首先,硝酸盐还原和铁氧化的耦合过程形成的三价铁矿物,能够改变营养物质或重金属的流动性进而影响局部环境[101-102]。其次,铁浓度可以直接影响多种氮循环步骤,例如生物可利用二价铁浓度的增加能加快河口沉积物中硝酸盐向氨的转化速率[由14.9升高至25.5 μmol Fe/(L·d),盐度为26.8 g/L],从而增加10%–20%的氮去除率,并降低反硝化速率[103]。此外,Hauck的研究显示,Lake Constance湖沉积物环境中的ANDFO微生物占总硝酸盐还原微生物的58%[104]。这也从另一方面说明了ANDFO过程是湖泊氮循环中不容忽视的一部分。
ANDFO过程受到硝酸根浓度、有机底物、磷酸根浓度等环境因素的影响。前人的实验表明,大多数混合营养型的ANDFO微生物需要一定浓度的有机底物来维持最佳的铁氧化速率[20, 95, 105-106];磷酸根会与亚铁形成磷酸亚铁沉淀(蓝铁矿),显著降低亚铁的生物可利用性,从而大幅降低ANDFO速率;而碳酸根在过饱和情况下虽然不会形成沉淀,也几乎不改变ANDFO速率,但会影响ANDFO过程最终形成的矿物种类[107]。
ANDFO过程会释放大量的自由能(503 kJ/(mol·N)),能帮助微生物抵御高渗透压胁迫[103]。Emmerich在一个超盐湖(盐度为348.6 g/L)沉积物中检测到210 cells/g(沉积物干重)硝酸盐还原铁氧化菌,说明ANDFO过程也许能够在接近盐度饱和的条件下发生[108]。以上发现均暗示着ANDFO过程可能具有一定的耐盐性,但未知其如何响应盐度变化。
2.4 硝酸盐还原硫氧化 除了有机碳和二价铁,硝酸盐还原菌还能够以硫化物为电子供体完成硝酸盐还原过程。在该过程中,硝酸根通过反硝化途径还原为氮气,硫化物可以被氧化为硫单质储存于细胞内或者进一步氧化为硫酸根。根据产物的不同,反应表达式不同(反应式4–5)。
 | 公式(4) |
 | 公式(5) |
硫化物丰富的海洋是最早发现硝酸盐还原硫氧化途径的生境。但前人研究显示,在淡水生态系统中该途径也扮演着重要的角色[21, 115-116]。硫化氢和硝酸根在水体中富集都会引起严重的环境问题,微生物介导的硝酸盐还原硫氧化反应能够同时去除水体中的氧化态氮和还原态硫,在维持水环境稳定方面具有重要的生态意义。例如,在西班牙三个淡水湖中,硝酸盐还原硫氧化途径对湖泊氮去除过程的贡献可达25%–40%;珠江河口沉积物环境中,硝酸盐还原硫氧化速率为0.196– 0.903 mmol/(L·h),在硝酸盐较低时(< 0.2 mmol/L),硝酸盐还原硫氧化途径对氮去除过程的贡献率则能够达到26%[21, 117]。
3 总结和展望 综合湖泊反硝化微生物群落与反硝化速率的研究发现,微生物介导的湖泊反硝化过程在减轻湖泊氮载荷方面起着不可忽视的作用,具有重要的生态环境意义。此外,微生物介导的反硝化与其他元素循环的耦合对于湖泊氮去除也有实质性贡献,拓宽了湖泊氮循环的研究思路。宏基因组、宏转录组、稳定同位素标记等新兴技术手段的出现与应用,帮助****们更清晰、准确地认识了反硝化微生物代谢机理和湖泊反硝化通量,也发现了一些亟待解决、值得深入探究的科学问题,例如:(1)人类活动引起全球氮沉降增加如何影响湖泊尤其是脆弱的高原湖泊反硝化微生物群落结构和功能;(2)目前的研究大多报道了某一种反硝化途径的脱氮速率与贡献率,缺乏对同一湖泊各种反硝化途径脱氮贡献的整体认识;(3)反硝化微生物与环境相互作用的生理机制尚不明确,有待更深入的研究;(4)氧化亚氮还原酶NOS比其他反硝化酶更敏感,那么在盐湖、碱湖中能否观察到N2O在反硝化终产物中占比升高,仍有待研究;(5)反硝化过程与其他元素循环耦合的酶学机制尚不明确,这一机制的突破将有助于****们利用基因手段发现和定量耦合反应的微生物群落,加快湖泊元素循环的研究进程。湖泊生境在全球氮循环中扮演着重要角色,湖泊生态功能与物质循环速率一直是国内外研究的热点话题。然而目前人类活动的加剧使得湖泊生态功能表现出退化的趋势,因此深入探究湖泊的物质循环规律能够为恢复湖泊生态功能、维持其生态稳定提供重要的参考意见。
References
| [1] | Seitzinger S, Harrison JA, B?hlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, van Drecht G. Denitrification across landscapes and waterscapes:a synthesis. Ecological Applications, 2006, 16(6): 2064-2090. DOI:10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2 |
| [2] | Gruber N, Galloway JN. An earth-system perspective of the global nitrogen cycle. Nature, 2008, 451(7176): 293-296. DOI:10.1038/nature06592 |
| [3] | Howarth RW, Marino R, Lane J, Cole JJ. Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1. Rates and importance. Limnology and Oceanography, 1988, 33(4): 669-687. |
| [4] | Seitzinger SP, Kroeze C. Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochemical Cycles, 1998, 12(1): 93-113. DOI:10.1029/97GB03657 |
| [5] | Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, V?osmarty CJ. Nitrogen cycles:past, present, and future. Biogeochemistry, 2004, 70(2): 153-226. DOI:10.1007/s10533-004-0370-0 |
| [6] | Cleveland CC, Houlton BZ, Smith WK, Marklein AR, Reed SC, Parton W, Del Grosso SJ, Running SW. Patterns of new versus recycled primary production in the terrestrial biosphere. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(31): 12733-12737. DOI:10.1073/pnas.1302768110 |
| [7] | Eugster O, Gruber N. A probabilistic estimate of global marine N-fixation and denitrification. Global Biogeochemical Cycles, 2012, 26(4): GB4013. |
| [8] | Gro?kopf T, Mohr W, Baustian T, Schunck H, Gill D, Kuypers MMM, Lavik G, Schmitz RA, Wallace DWR, LaRoche J. Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature, 2012, 488(7411): 361-364. DOI:10.1038/nature11338 |
| [9] | Battye W, Aneja VP, Schlesinger WH. Is nitrogen the next carbon?. Earth's Future, 2017, 5(9): 894-904. DOI:10.1002/2017EF000592 |
| [10] | Herridge DF, Peoples MB, Boddey RM. Global inputs of biological nitrogen fixation in agricultural systems. Plant and Soil, 2008, 311(1/2): 1-18. |
| [11] | Maavara T, Lauerwald R, Laruelle GG, Akbarzadeh Z, Bouskill NJ, van Cappellen P, Regnier P. Nitrous oxide emissions from inland waters:Are IPCC estimates too high?. Global Change Biology, 2019, 25(2): 473-488. DOI:10.1111/gcb.14504 |
| [12] | Codispoti LA, Brandes JA, Christensen JP, Devol AH, Naqvi SWA, Paerl HW, Yoshinari T. The oceanic fixed nitrogen and nitrous oxide budgets:Moving targets as we enter the anthropocene?. Scientia Marina, 2001, 65(S2): 85-105. DOI:10.3989/scimar.2001.65s285 |
| [13] | Yu GR, Jia YL, He NP, Zhu JX, Chen Z, Wang QF, Piao SL, Liu XJ, He HL, Guo XB, Wen Z, Li P, Ding GA, Goulding K. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nature Geoscience, 2019, 12: 424-429. DOI:10.1038/s41561-019-0352-4 |
| [14] | Elser JJ, Andersen T, Baron JS, Bergstr?m AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science, 2009, 326(5954): 835-837. DOI:10.1126/science.1176199 |
| [15] | Seitzinger SP. Denitrification in freshwater and coastal marine ecosystems:ecological and geochemical significance. Limnology and Oceanography, 1988, 33(4): 702-724. |
| [16] | Ravishankara A, Daniel JS, Portmann RW. Nitrous oxide (N2O):the dominant ozone-depleting substance emitted in the 21st century. Science, 2009, 326(5949): 123-125. DOI:10.1126/science.1176985 |
| [17] | W MO. The state of greenhouse gases in the atmosphere based on global observations through 2012. WMO Greenhouse Gas Bulletin, 2013, 9: 1-4. |
| [18] | Spott O, Russow R, Stange CF. Formation of hybrid N2O and hybrid N2 due to codenitrification:First review of a barely considered process of microbially mediated N-nitrosation. Soil Biology and Biochemistry, 2011, 43(10): 1995-2011. DOI:10.1016/j.soilbio.2011.06.014 |
| [19] | Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM, Schreiber F, Dutilh BE, Zedelius J, De Beer D, Gloerich J, Wessels HJCT, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJM, Janssen-Megens EM, Francoijs KJ, Stunnenberg H, Weissenbach J, Jetten MSM, Strous M. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature, 2010, 464(7288): 543-548. DOI:10.1038/nature08883 |
| [20] | Straub KL, Benz M, Schink B, Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Applied and Environmental Microbiology, 1996, 62(4): 1458-1460. DOI:10.1128/AEM.62.4.1458-1460.1996 |
| [21] | Burgin AJ, Hamilton SK. NO3?-driven SO42? production in freshwater ecosystems:implications for N and S cycling. Ecosystems, 2008, 11(6): 908-922. DOI:10.1007/s10021-008-9169-5 |
| [22] | Canfield DE, Thamdrup B, Kristensen E. Aquatic geomicrobiology. Amsterdam: Academic Press, 2005. |
| [23] | Zumft WG. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews, 1997, 61(4): 533-616. |
| [24] | Xia XH, Zhang SB, Li SL, Zhang LW, Wang GQ, Zhang L, Wang JF, Li ZH. The cycle of nitrogen in river systems:sources, transformation, and flux. Environmental Science:Processes & Impacts, 2018, 20(6): 863-891. |
| [25] | Burgin AJ, Hamilton SK. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment, 2007, 5(2): 89-96. DOI:10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2 |
| [26] | Lam P, Kuypers MMM. Microbial nitrogen cycling processes in oxygen minimum zones. Annual Review of Marine Science, 2011, 3(1): 317-345. DOI:10.1146/annurev-marine-120709-142814 |
| [27] | Kraft B, Tegetmeyer HE, Sharma R, Klotz MG, Ferdelman TG, Hettich RL, Geelhoed JS, Strous M. The environmental controls that govern the end product of bacterial nitrate respiration. Science, 2014, 345(6197): 676-679. DOI:10.1126/science.1254070 |
| [28] | Maia LB, Moura JJG. How biology handles nitrite. Chemical Reviews, 2014, 114(10): 5273-5357. DOI:10.1021/cr400518y |
| [29] | Graf DRH, Jones CM, Hallin S. Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PLoS One, 2014, 9(12): e114118. DOI:10.1371/journal.pone.0114118 |
| [30] | Fang FC. Antimicrobial reactive oxygen and nitrogen species:concepts and controversies. Nature Reviews Microbiology, 2004, 2(10): 820-832. DOI:10.1038/nrmicro1004 |
| [31] | Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science, 2010, 330(6011): 1666-1670. DOI:10.1126/science.1195591 |
| [32] | Matsumoto Y, Tosha T, Pisliakov AV, Hino T, Sugimoto H, Nagano S, Sugita Y, Shiro Y. Crystal structure of quinol-dependent nitric oxide reductase from Geobacillus stearothermophilus. Nature Structural & Molecular Biology, 2012, 19(2): 238-245. |
| [33] | Al-Attar S, de Vries S. An electrogenic nitric oxide reductase. FEBS Letters, 2015, 589(16): 2050-2057. DOI:10.1016/j.febslet.2015.06.033 |
| [34] | McCrackin ML, Elser JJ. Greenhouse gas dynamics in lakes receiving atmospheric nitrogen deposition. Global Biogeochemical Cycles, 2011, 25(4): GB4005. |
| [35] | Kroeze C, Dumont E, Seitzinger S. Future trends in emissions of N2O from rivers and estuaries. Journal of Integrative Environmental Sciences, 2010, 7(S1): 71-78. |
| [36] | Whitfield CJ, Aherne J, Baulch HM. Controls on greenhouse gas concentrations in polymictic headwater lakes in Ireland. Science of the Total Environment, 2011, 410-411: 217-225. DOI:10.1016/j.scitotenv.2011.09.045 |
| [37] | Huttunen JT, Alm J, Liikanen A, Juutinen S, Larmola T, Hammar T, Silvola J, Martikainen PJ. Fluxes of methane, carbon dioxide and nitrous oxide in boreal lakes and potential anthropogenic effects on the aquatic greenhouse gas emissions. Chemosphere, 2003, 52(3): 609-621. DOI:10.1016/S0045-6535(03)00243-1 |
| [38] | McCrackin ML, Elser JJ. Atmospheric nitrogen deposition influences denitrification and nitrous oxide production in lakes. Ecology, 2010, 91(2): 528-539. |
| [39] | Chen H, Wang M, Wu N, Wang YF, Zhu D, Gao YH, Peng CH. Nitrous oxide fluxes from the littoral zone of a lake on the Qinghai-Tibetan Plateau. Environmental Monitoring and Assessment, 2011, 182(1/4): 545-553. |
| [40] | Downes M. The production and consumption of nitrate in an eutrophic lake during early stratification. Archiv für Hydrobiologie, 1991, 122(3): 257-274. |
| [41] | Wang SL, Liu CQ, Yeager KM, Wan GJ, Li J, Tao FX, Lü YC, Liu F, Fan CX. The spatial distribution and emission of nitrous oxide (N2O) in a large eutrophic lake in eastern China:anthropogenic effects. Science of the Total Environment, 2009, 407(10): 3330-3337. DOI:10.1016/j.scitotenv.2008.10.037 |
| [42] | Fuchs VJ, Mihelcic JR, Gierke JS. Life cycle assessment of vertical and horizontal flow constructed wetlands for wastewater treatment considering nitrogen and carbon greenhouse gas emissions. Water Research, 2011, 45(5): 2073-2081. DOI:10.1016/j.watres.2010.12.021 |
| [43] | Nielsen M, Gieseke A, de Beer D, Revsbech NP. Nitrate, nitrite, and nitrous oxide transformations in sediments along a salinity gradient in the Weser Estuary. Aquatic Microbial Ecology, 2009, 55: 39-52. DOI:10.3354/ame01275 |
| [44] | Frame CH, Lau E, Nolan EJ IV, Goepfert TJ, Lehmann MF. Acidification enhances hybrid N2O production associated with aquatic ammonia-oxidizing microorganisms. Frontiers in Microbiology, 2016, 7: 2104. |
| [45] | Von Schulthess R, Wild D, Gujer W. Nitric and nitrous oxides from denitrifying activated sludge at low oxygen concentration. Water Science and Technology, 1994, 30(6): 123-132. DOI:10.2166/wst.1994.0259 |
| [46] | Hunt PG, Matheny TA, Ro KS. Nitrous oxide accumulation in soils from riparian buffers of a coastal plain watershed-carbon/nitrogen ratio control. Journal of Environment Quality, 2007, 36(5): 1368-1376. DOI:10.2134/jeq2006.0255 |
| [47] | Inamori R, Wang YH, Yamamoto T, Zhang JX, Kong HN, Xu KQ, Inamori Y. Seasonal effect on N2O formation in nitrification in constructed wetlands. Chemosphere, 2008, 73(7): 1071-1077. DOI:10.1016/j.chemosphere.2008.07.064 |
| [48] | Maltais-Landry G, Maranger R, Brisson J, Chazarenc F. Greenhouse gas production and efficiency of planted and artificially aerated constructed wetlands. Environmental Pollution, 2009, 157(3): 748-754. DOI:10.1016/j.envpol.2008.11.019 |
| [49] | Cabello P, Roldán MD, Moreno-Vivián C. Nitrate reduction and the nitrogen cycle in archaea. Microbiology, 2004, 150(11): 3527-3546. DOI:10.1099/mic.0.27303-0 |
| [50] | Zumft WG, Kroneck PMH. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by Bacteria and Archaea. Advances in Microbial Physiology, 2006, 52: 107-227. DOI:10.1016/S0065-2911(06)52003-X |
| [51] | Hallin S, Philippot L, L?ffler FE, Sanford RA, Jones CM. Genomics and ecology of novel N2O-reducing microorganisms. Trends in Microbiology, 2018, 26(1): 43-55. DOI:10.1016/j.tim.2017.07.003 |
| [52] | Jones CM, Spor A, Brennan FP, Breuil MC, Bru D, Lemanceau P, Griffiths B, Hallin S, Philippot L. Recently identified microbial guild mediates soil N2O sink capacity. Nature Climate Change, 2014, 4(9): 801-805. DOI:10.1038/nclimate2301 |
| [53] | Philippot L, Andert J, Jones CM, Bru D, Hallin S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Global Change Biology, 2011, 17(3): 1497-1504. DOI:10.1111/j.1365-2486.2010.02334.x |
| [54] | Soued C, Del Giorgio PA, Maranger R. Nitrous oxide sinks and emissions in boreal aquatic networks in Québec. Nature Geoscience, 2016, 9(2): 116-120. DOI:10.1038/ngeo2611 |
| [55] | Lu XX, Bade DL, Leff LG, Mou XZ. The relative importance of anammox and denitrification to total N2 production in Lake Erie. Journal of Great Lakes Research, 2018, 44(3): 428-435. DOI:10.1016/j.jglr.2018.03.008 |
| [56] | Yao L, Jiang XL, Chen CR, Liu GH, Liu WZ. Within-lake variability and environmental controls of sediment denitrification and associated N2O production in a shallow eutrophic lake. Ecological Engineering, 2016, 97: 251-257. DOI:10.1016/j.ecoleng.2016.10.023 |
| [57] | Yao XL, Zhang L, Zhang YL, Xu HX, Jiang XY. Denitrification occurring on suspended sediment in a large, shallow, subtropical lake (Poyang Lake, China). Environmental Pollution, 2016, 219: 501-511. DOI:10.1016/j.envpol.2016.05.073 |
| [58] | Yao L, Chen CR, Liu GH, Liu WZ. Sediment nitrogen cycling rates and microbial abundance along a submerged vegetation gradient in a eutrophic lake. Science of the Total Environment, 2018, 616-617: 899-907. DOI:10.1016/j.scitotenv.2017.10.230 |
| [59] | Liu WZ, Yao L, Jiang XL, Guo LD, Cheng XL, Liu GH. Sediment denitrification in Yangtze lakes is mainly influenced by environmental conditions but not biological communities. Science of the Total Environment, 2018, 616-617: 978-987. DOI:10.1016/j.scitotenv.2017.10.221 |
| [60] | Saunders DL, Kalff J. Nitrogen retention in wetlands, lakes and rivers. Hydrobiologia, 2001, 443(1/3): 205-212. DOI:10.1023/A:1017506914063 |
| [61] | Liaw A, Wiener M. Classification and regression by randomForest. R News, 2002, 2-3: 18-22. |
| [62] | Pi?a-Ochoa E, álvarez-Cobelas M. Denitrification in aquatic environments:a cross-system analysis. Biogeochemistry, 2006, 81(1): 111-130. DOI:10.1007/s10533-006-9033-7 |
| [63] | Magalh?es CM, Joye SB, Moreira RM, Wiebe WJ, Bordalo AA. Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Research, 2005, 39(9): 1783-1794. DOI:10.1016/j.watres.2005.03.008 |
| [64] | Din?er A, Kargi F. Salt inhibition of nitrification and denitrification in saline wastewater. Environmental Technology, 1999, 20(11): 1147-1153. DOI:10.1080/09593332008616912 |
| [65] | Mancinelli RL, Hochstein LI. The occurrence of denitrification in extremely halophilic bacteria. FEMS Microbiology Letters, 1986, 35(1): 55-58. DOI:10.1111/j.1574-6968.1986.tb01498.x |
| [66] | Oren A. Thermodynamic limits to microbial life at high salt concentrations. Environmental Microbiology, 2011, 13(8): 1908-1923. DOI:10.1111/j.1462-2920.2010.02365.x |
| [67] | Spott O, Florian Stange C. Formation of hybrid N2O in a suspended soil due to co-denitrification of NH2OH. Journal of Plant Nutrition and Soil Science, 2011, 174(4): 554-567. DOI:10.1002/jpln.201000200 |
| [68] | Medinets S, Skiba U, Rennenberg H, Butterbach-Bahl K. A review of soil NO transformation:associated processes and possible physiological significance on organisms. Soil Biology and Biochemistry, 2015, 80: 92-117. DOI:10.1016/j.soilbio.2014.09.025 |
| [69] | Tanimoto T, Hatano KI, Kim DH, Uchiyama H, Shoun H. Co-denitrification by the denitrifying system of the fungus Fusarium oxysporum. FEMS Microbiology Letters, 1992, 93(2): 177-180. DOI:10.1111/j.1574-6968.1992.tb05086.x |
| [70] | Okada N, Nomura N, Nakajima-Kambe T, Uchiyama H. Characterization of the aerobic denitrification in Mesorhizobium sp. strain NH-14 in comparison with that in related rhizobia. Microbes and Environments, 2005, 20(4): 208-215. DOI:10.1264/jsme2.20.208 |
| [71] | Su F, Takaya N, Shoun H. Nitrous oxide-forming codenitrification catalyzed by cytochrome P450nor. Bioscience, Biotechnology, and Biochemistry, 2004, 68(2): 473-475. DOI:10.1271/bbb.68.473 |
| [72] | Immoos CE, Chou J, Bayachou M, Blair E, Greaves J, Farmer PJ. Electrocatalytic reductions of nitrite, nitric oxide, and nitrous oxide by thermophilic cytochrome P450 CYP119 in film-modified electrodes and an analytical comparison of its catalytic activities with myoglobin. Journal of the American Chemical Society, 2004, 126(15): 4934-4942. DOI:10.1021/ja038925c |
| [73] | Laughlin RJ, Stevens RJ. Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Science Society of America Journal, 2002, 66(5): 1540-1548. DOI:10.2136/sssaj2002.1540 |
| [74] | Bol R, R?ckmann T, Blackwell M, Yamulki S. Influence of flooding on δ15N, δ18O, 1δ15N and 2δ15N signatures of N2O released from estuarine soils-a laboratory experiment using tidal flooding chambers. Rapid Communications in Mass Spectrometry, 2004, 18(14): 1561-1568. DOI:10.1002/rcm.1519 |
| [75] | Erler DV, Eyre BD, Davison L. The contribution of anammox and denitrification to sediment N2 production in a surface flow constructed wetland. Environmental Science & Technology, 2008, 42(24): 9144-9150. |
| [76] | Münster U. Concentrations and fluxes of organic carbon substrates in the aquatic environment. Antonie van Leeuwenhoek, 1993, 63(3/4): 243-274. |
| [77] | Lee C. Amino acid and amine biogeochemistry in marine particulate material and sediments//Blackburn TH, S?renson J. Nitrogen Cycling in Coastal Marine Environments. New York: Wiley & Sons, 1988: 125-141. |
| [78] | Raghoebarsing AA, Pol A, Van de Pas-Schoonen KT, Smolders AJP, Ettwig KF, Rijpstra WIC, Schouten S, Damsté JSS, Op den Camp HJM, Jetten MSM, Strous M. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature, 2006, 440(7086): 918-921. DOI:10.1038/nature04617 |
| [79] | Haroon MF, Hu SH, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan ZG, Tyson GW. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature, 2013, 500(7464): 567-570. DOI:10.1038/nature12375 |
| [80] | Ettwig KF, Shima S, Van De Pas-Schoonen KT, Kahnt J, Medema MH, Op Den Camp HJM, Jetten MSM, Strous M. Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environmental Microbiology, 2008, 10(11): 3164-3173. DOI:10.1111/j.1462-2920.2008.01724.x |
| [81] | Brune A, Frenzel P, Cypionka H. Life at the oxic-anoxic interface:microbial activities and adaptations. FEMS Microbiology Reviews, 2000, 24(5): 691-710. DOI:10.1111/j.1574-6976.2000.tb00567.x |
| [82] | Deutzmann JS, Schink B. Anaerobic oxidation of methane in sediments of Lake Constance, an oligotrophic freshwater lake. Applied and Environmental Microbiology, 2011, 77(13): 4429-4436. DOI:10.1128/AEM.00340-11 |
| [83] | Norei K, Thamdrup B. Nitrate-dependent anaerobic methane oxidation in a freshwater sediment. Geochimica et Cosmochimica Acta, 2014, 132: 141-150. DOI:10.1016/j.gca.2014.01.032 |
| [84] | Shen LD, Huang Q, He ZF, Lian X, Liu S, He YF, Lou LP, Xu XY, Zheng P, Hu BL. Vertical distribution of nitrite-dependent anaerobic methane-oxidising bacteria in natural freshwater wetland soils. Applied Microbiology and Biotechnology, 2015, 99(1): 349-357. DOI:10.1007/s00253-014-6031-x |
| [85] | Zhang MP, Luo Y, Lin LA, Lin XL, Hetharua B, Zhao WJ, Zhou MK, Zhan Q, Xu H, Zheng TL, Tian Y. Molecular and stable isotopic evidence for the occurrence of nitrite-dependent anaerobic methane-oxidizing bacteria in the mangrove sediment of Zhangjiang Estuary, China. Applied Microbiology and Biotechnology, 2018, 102(5): 2441-2454. DOI:10.1007/s00253-017-8718-2 |
| [86] | Deutzmann JS, Stief P, Brandes J, Schink B. Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(51): 18273-18278. DOI:10.1073/pnas.1411617111 |
| [87] | He ZF, Cai C, Shen LD, Lou LP, Zheng P, Xu XH, Hu BL. Effect of inoculum sources on the enrichment of nitrite-dependent anaerobic methane-oxidizing bacteria. Applied Microbiology and Biotechnology, 2015, 99(2): 939-946. DOI:10.1007/s00253-014-6033-8 |
| [88] | Chen J, Zhou ZC, Gu JD. Occurrence and diversity of nitrite-dependent anaerobic methane oxidation bacteria in the sediments of the South China Sea revealed by amplification of both 16S rRNA and pmoA genes. Applied Microbiology and Biotechnology, 2014, 98(12): 5685-5696. DOI:10.1007/s00253-014-5733-4 |
| [89] | Borrel G, Jézéquel D, Biderre-Petit C, Morel-Desrosiers N, Morel JP, Peyret P, Fonty G, Lehours AC. Production and consumption of methane in freshwater lake ecosystems. Research in Microbiology, 2011, 162(9): 832-847. DOI:10.1016/j.resmic.2011.06.004 |
| [90] | Zhang XW, Liu Y, Gu JD. A global analysis on the distribution pattern of the bacteria coupling simultaneous methane oxidation to nitrite reduction. International Biodeterioration & Biodegradation, 2018, 129: 123-132. |
| [91] | Yang J, Jiang HC, Wu G, Hou WG, Sun YJ, Lai ZP, Dong HL. Co-occurrence of nitrite-dependent anaerobic methane oxidizing and anaerobic ammonia oxidizing bacteria in two Qinghai-Tibetan saline lakes. Frontiers of Earth Science, 2012, 6(4): 383-391. |
| [92] | Chen J, Zhou ZC, Gu JD. Complex community of nitrite-dependent anaerobic methane oxidation bacteria in coastal sediments of the Mai Po wetland by PCR amplification of both 16S rRNA and pmoA genes. Applied Microbiology and Biotechnology, 2015, 99(3): 1463-1473. DOI:10.1007/s00253-014-6051-6 |
| [93] | Li QQ, Wang FP, Chen ZW, Yin XJ, Xiao X. Stratified active archaeal communities in the sediments of Jiulong River estuary, China. Frontiers in Microbiology, 2012, 3: 311. |
| [94] | Hafenbradl D, Keller M, Dirmeier R, Rachel R, Ro?nagel P, Burggraf S, Huber H, Stetter KO. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Archives of Microbiology, 1996, 166(5): 308-314. DOI:10.1007/s002030050388 |
| [95] | Schaedler F, Lockwood C, Lueder U, Glombitza C, Kappler A, Schmidt C. Microbially mediated coupling of Fe and N cycles by nitrate-reducing Fe(Ⅱ)-oxidizing bacteria in littoral freshwater sediments. Applied and Environmental Microbiology, 2018, 84(2): e02013-17. |
| [96] | Melton ED, Schmidt C, Kappler A. Microbial iron(Ⅱ) oxidation in littoral freshwater lake sediment:the potential for competition between phototrophic vs. nitrate-reducing iron(Ⅱ)-oxidizers. Frontiers in Microbiology, 2012, 3: 197. |
| [97] | Chakraborty A, Picardal F. Induction of nitrate-dependent Fe(Ⅱ) oxidation by Fe(Ⅱ) in Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Applied and Environmental Microbiology, 2013, 79(2): 748-752. |
| [98] | Hedrich S, Schl?mann M, Johnson DB. The iron-oxidizing proteobacteria. Microbiology, 2011, 157(6): 1551-1564. DOI:10.1099/mic.0.045344-0 |
| [99] | Weber KA, Pollock J, Cole KA, O'Connor SM, Achenbach LA, Coates JD. Anaerobic nitrate-dependent iron(Ⅱ) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Applied and Environmental Microbiology, 2006, 72(1): 686-694. |
| [100] | He SM, Tominski C, Kappler A, Behrens S, Roden EE. Metagenomic analyses of the autotrophic Fe(Ⅱ)-oxidizing, nitrate-reducing enrichment culture KS. Applied and Environmental Microbiology, 2016, 82(9): 2656-2668. DOI:10.1128/AEM.03493-15 |
| [101] | Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron:anaerobic microbial iron oxidation and reduction. Nature Reviews Microbiology, 2006, 4(10): 752-764. DOI:10.1038/nrmicro1490 |
| [102] | Lack JG, Chaudhuri SK, Kelly SD, Kemner KM, O'Connor SM, Coates JD. Immobilization of radionuclides and heavy metals through anaerobic bio-oxidation of Fe(Ⅱ). Applied and Environmental Microbiology, 2002, 68(6): 2704-2710. DOI:10.1128/AEM.68.6.2704-2710.2002 |
| [103] | Robertson EK, Roberts KL, Burdorf LDW, Cook P, Thamdrup B. Dissimilatory nitrate reduction to ammonium coupled to Fe(Ⅱ) oxidation in sediments of a periodically hypoxic estuary. Limnology and Oceanography, 2016, 61(1): 365-381. DOI:10.1002/lno.10220 |
| [104] | Hauck S, Benz M, Brune A, Schink B. Ferrous iron oxidation by denitrifying bacteria in profundal sediments of a deep lake (Lake Constance). FEMS Microbiology Ecology, 2001, 37(2): 127-134. DOI:10.1111/j.1574-6941.2001.tb00860.x |
| [105] | Chaudhuri SK, Lack JG, Coates JD. Biogenic magnetite formation through anaerobic biooxidation of Fe(Ⅱ). Applied and Environmental Microbiology, 2001, 67(6): 2844-2848. DOI:10.1128/AEM.67.6.2844-2848.2001 |
| [106] | Laufer K, Byrne JM, Glombitza C, Schmidt C, J?rgensen BB, Kappler A. Anaerobic microbial Fe(Ⅱ) oxidation and Fe(Ⅲ) reduction in coastal marine sediments controlled by organic carbon content. Environmental Microbiology, 2016, 18(9): 3159-3174. DOI:10.1111/1462-2920.13387 |
| [107] | Larese-Casanova P, Haderlein SB, Kappler A. Biomineralization of lepidocrocite and goethite by nitrate-reducing Fe(Ⅱ)-oxidizing bacteria:effect of pH, bicarbonate, phosphate, and humic acids. Geochimica et Cosmochimica Acta, 2010, 74(13): 3721-3734. DOI:10.1016/j.gca.2010.03.037 |
| [108] | Emmerich M, Bhansali A, L?sekann-Behrens T, Schr?der C, Kappler A, Behrens S. Abundance, distribution, and activity of Fe(Ⅱ)-oxidizing and Fe(Ⅲ)-reducing microorganisms in hypersaline sediments of Lake Kasin, southern Russia. Applied and Environmental Microbiology, 2012, 78(12): 4386-4399. DOI:10.1128/AEM.07637-11 |
| [109] | Timmer-Ten Hoor A. A new type of thiosulphate oxidizing, nitrate reducing microorganism:Thiomicrospira denitrificans sp. nov. Netherlands Journal of Sea Research, 1975, 9(3/4): 344-350. |
| [110] | Sorokin DY. Diversity of halophilic sulfur-oxidizing bacteria in hypersaline habitats//Dahl C, Friedrich CG. Microbial Sulfur Metabolism. Berlin, Heidelberg: Springer, 2008: 225-237. |
| [111] | Sorokin DY, Kuenen JG, Jetten MSM. Denitrification at extremely high pH values by the alkaliphilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium Thioalkalivibrio denitrificans strain ALJD. Archives of Microbiology, 2001, 175(2): 94-101. DOI:10.1007/s002030000210 |
| [112] | Sorokin DY, Tourova TP, Galinski EA, Muyzer G, Kuenen JG. Thiohalorhabdus denitrificans gen. nov., sp. nov., an extremely halophilic, sulfur-oxidizing, deep-lineage gammaproteobacterium from hypersaline habitats. International Journal of Systematic and Evolutionary Microbiology, 2008, 58(12): 2890-2897. DOI:10.1099/ijs.0.2008/000166-0 |
| [113] | Sorokin DY, Lysenko AM, Mityushina LL, Tourova TP, Jones BE, Rainey FA, Robertson LA, Kuenen GJ. Thioalkalimicrobium aerophilum gen. nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. nov., sp. nov., Thioalkalivibrio nitratis sp. nov., novel and Thioalkalivibrio denitrificancs sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. International Journal of Systematic and Evolutionary Microbiology, 2001, 51(2): 565-580. DOI:10.1099/00207713-51-2-565 |
| [114] | Wang XN, Sun GX, Zhu YG. Thermodynamic energy of anaerobic microbial redox reactions couples elemental biogeochemical cycles. Journal of Soils and Sediments, 2017, 17(12): 2831-2846. DOI:10.1007/s11368-017-1767-4 |
| [115] | Fossing H, Gallardo VA, J?rgensen BB, Hüttel M, Nielsen LP, Schulz H, Canfield DE, Forster S, Glud RN, Gundersen JK, Küver J, Ramsing NB, Teske A, Thamdrup B, Ulloa O. Concentration and transport of nitrate by the mat-forming sulphur bacterium Thioploca. Nature, 1995, 374(6524): 713-715. DOI:10.1038/374713a0 |
| [116] | Payne E, Burgin AJ, Hamilton SK. Sediment nitrate manipulation using porewater equilibrators reveals potential for N and S coupling in freshwaters. Aquatic Microbial Ecology, 2009, 54(3): 233-241. |
| [117] | Yang XN, Huang S, Wu QH, Zhang RD. Nitrate reduction coupled with microbial oxidation of sulfide in river sediment. Journal of Soils and Sediments, 2012, 12(9): 1435-1444. DOI:10.1007/s11368-012-0542-9 |
