陈梅春1, 王阶平1, 肖荣凤1, 刘波1

 , 刘晓港2, 葛慈斌1, 阮传清1, 朱育菁1
, 刘晓港2, 葛慈斌1, 阮传清1, 朱育菁1 1.福建省农业科学院农业生物资源研究所, 福建 福州 350003;
2.福州大学生物科学与工程学院, 福建 福州 350108
收稿日期:2017-04-10;修回日期:2017-05-31;网络出版日期:2017-07-11
基金项目:国家自然科学基金(31370059);福建省农业科学院英才计划(YC2015-11)
*通信作者:刘波, Tel/Fax:+86-591-87882571;E-mail:fzliubo@163.com
摘要:[目的]地衣芽胞杆菌FJAT-4产生的脂肽能够有效抑制尖孢镰刀菌的生长,本研究的目的在于探究地衣芽胞杆菌FJAT-4脂肽结构,分析培养基组分和培养温度对FJAT-4产抑菌脂肽的影响,阐述脂肽对尖孢镰刀菌的抑制作用,为菌株抑菌机理的阐释及其在枯萎病防治中的推广应用奠定基础。[方法]通过酸沉醇提法提取地衣芽胞杆菌FJAT-4产生的脂肽;利用液相色谱串联四极杆飞行时间质谱进行地衣芽胞杆菌FJAT-4脂肽组成分析及结构鉴定;以抑菌圈大小为指标评估地衣芽胞杆菌FJAT-4脂肽对尖孢镰刀菌的抑制效果;通过扫描电镜观察地衣芽胞杆菌FJAT-4粗脂肽对尖孢镰刀菌的抑制作用。[结果]地衣芽胞杆菌FJAT-4产生的抑菌脂肽由C17fengycin A、C17fengycin B、C17fengycin B2、C16fengycin A衍生物、C16fengycin B衍生物、C13–C15surfactin及C13–C15surfactin衍生物组成,其中C13–C15 surfactin衍生物(m/z [M+Na]+=1048.6/1062.6/1076.6)为新化合物。培养基成分不同对菌株FJAT-4脂肽组成影响较小,但温度对菌株FJAT-4产生抑菌脂肽的影响很大,该菌株在较低温度(20–25 ℃)下培养不产生脂肽,30–40 ℃下培养能产生抑菌脂肽,且高温有利于提高脂肽中surfactin的比例。该脂肽类物质对辣椒、番茄、香蕉和甜瓜尖孢镰刀菌等多种植物病原真菌均具有很好的抑制效果,且呈剂量依赖性。扫描电镜结果表明地衣芽胞杆菌FJAT-4所产的脂肽会严重影响辣椒、番茄、香蕉和甜瓜尖孢镰刀菌菌丝的正常生长,导致菌丝断裂变形、孢子变形或显著抑制了孢子的生长。[结论]地衣芽胞杆菌FJAT-4产生的抑菌脂肽为fengycin和surfactin类物质,该抑菌脂肽会致使尖孢镰刀菌菌丝体发育畸形,影响尖孢镰刀菌的正常生长。
关键词: 地衣芽胞杆菌 尖孢镰刀菌 脂肽 液相色谱串联四极杆飞行时间质谱
Characterization of lipopeptides produced by Bacillus licheniformis FJAT-4 and their effect on Fusarium oxysporum
Meichun Chen1, Jieping Wang1, Rongfeng Xiao1, Bo Liu1

 , Xiaogang Liu2, Cibin Ge1, Chuanqing Ruan1, Yujing Zhu1
, Xiaogang Liu2, Cibin Ge1, Chuanqing Ruan1, Yujing Zhu1 1.Agricultural Bioresources Research Institute, Fujian Academy of Agricultural Sciences, Fuzhou 350003, Fujian Province, China;
2.College of Biological Science and Engineering, Fuzhou University, Fuzhou 350108, Fujian Province, China
Received 10 April 2017; Revised 31 May 2017; Published online 11 July 2017
*Corresponding author: Bo Liu, Tel/Fax:+86-591-87882571;E-mail:fzliubo@163.com
Supported by the National Natural Science Foundation of China (31370059) and by the Youth Fund of FAAS (YC2015-11)
Abstract: [Objective]Lipopeptides produced by Bacillus licheniformis FJAT-4 can strongly inhibit the mycelia growth of Fusarium oxysporum. The aim of this study was to identify the structure of antifungal lipopeptide, analyze the effect of culture medium and temperature on the antifungal lipopeptide production, and explain antifungal effect of lipopeptide against F. oxysporum. This study is helpful to understand the mechanisms of Bacillus licheniformis FJAT-4 in controlling F. oxysporum.[Methods]Crude lipopeptides were extracted by acid precipitation and resolved in methanol. Their structures were identified using liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Their antifungal activities were determined through the assay of inhibition zone experiment. Their effects against F. oxysporum were observed using scanning electron microscopy.[Results]Lipopeptides produced by B. licheniformis FJAT-4 were identified as C17fengycin A, C17fengycin B, C17fengycin B2, C16fengycin A variant, C16fengycin B variant, C13–C15surfactin and C13–C15surfactin variants. Among them, C13–C15surfactin variants with m/z 1048.6/1062.6/1076.6 were new compounds. Results showed that the culture mediums have relatively little influence on the composition of lipopeptides produced by FJAT-4. However, the culture temperature presented obviously impact on the production of antifungal lipopeptides, which can only be produced by strain FJAT-4 growing at higher temperature (30–40 ℃) in comparison to temperature between 20–25 ℃. The higher culture temperature was in favour of increasing the proportion of surfactins in lipopeptide mixture. The lipopeptides exhibited strong antifungal activity against several plant pathogenic F. oxysporum strains in a dosage-dependent mode. Observation under scanning electron microscopy showed that the antagonistic lipopeptides secreted by B. licheniformis FJAT-4 significantly led to abnormal mycelia and spore growth of F. oxysporum.[Conclusion]The lipopeptides produced by FJAT-4 was composed of fengycins and surfactins. These lipopeptides display strong antifungal activity against several strains of plant pathogenic F. oxysporum, and destroyed the mycelial structure of the pathogenic F. oxysporum.
Key words: Bacillus licheniformis Fusarium oxysporum lipopeptides liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry
芽胞杆菌是一类重要的植物病虫害生防菌,由芽胞杆菌产生的抑菌脂肽,具有广谱抗菌活性、不易产生耐药性、稳定性好等优点,是潜在的传统抗生素理想替代品之一[1-2]。长期以来,芽胞杆菌用于防治生物病害,是以其发酵的活菌体为指标,有效成分和作用机理不明确,且活菌受环境条件影响较大,导致田间防治效果存在不稳定性。研究芽胞杆菌抑菌脂肽成分及作用机理对于了解生防菌的有效成分和生防机理,提高芽胞杆菌生防菌防治效果和稳定性具有重要意义。
目前已报道可产脂肽的芽胞杆菌主要包括枯草芽胞杆菌、解淀粉芽胞杆菌、苏云金芽胞杆菌、甲基营养型芽胞杆菌等[3-7]。芽胞杆菌脂肽是利用多载体巯基化模板机制,通过非核糖体多肽合成酶合成的[8]。芽胞杆菌产生的脂肽包括iturin (伊枯草菌素)、fengycin (丰原素)和surfactin (表面活性素)等。Iturin由七肽和β-NH2脂肪酸链组成,包括iturin、mycosubtilin、bacillomycin等[9-12]。Iturin是一种生物表面活性剂,可以将水的表面张力由72 mN/m降至43 mN/m,能够强烈抑制酵母菌和真菌,但对细菌的抑菌作用很微弱,其作用机制较为复杂[9-13]。Surfactin家族具有20余种同系物,除了埃斯波素(esperin)外,都是由7个氨基酸肽链与β-羟基脂肪酸交联形成的内酯环状结构,surfactin是比iturin更强的生物表面活性剂,可以降低水面张力至27 mN/m,具有强乳化作用,可以溶解和破坏磷脂膜,能够破坏细菌和有脂膜包被的病毒,但对真菌抗性不佳[14-16]。Fengycin家族由10个氨基酸链和β-羟基脂肪酸链连接形成的,包括fengycin A、B,plipastatin A1、A2、B1和B2,其对丝状真菌有很好的抑制作用,而对酵母和细菌无作用,其抑菌作用机理可能是改变病原菌细胞膜通透性或破坏细胞膜结构[17-19]。
目前关于芽胞杆菌抑菌脂肽的研究主要集中在枯草芽胞杆菌和解淀粉芽胞杆菌,对地衣芽胞杆菌抑菌脂肽的研究报道较少。前期实验室已获得一株对枯萎病具有良好抑制作用的菌株FJAT-4,进行了生物学特性、发酵工艺和田间应用等研究,该菌株发酵液对尖孢镰刀菌的抑菌率达80.9%[20-21],但抑菌活性物质还不明确。前期研究表明,该菌株分泌的脂肽类物质能有效抑制尖孢镰刀菌的生长。因此,本研究围绕新型脂肽鉴定、发酵培养条件对抑菌脂肽的影响以及脂肽对尖孢镰刀菌的抑制作用开展研究,通过液相色谱串联四极杆飞行时间质谱(LC-QTOF-MS/MS)对地衣芽胞杆菌FJAT-4产生的抑菌脂肽组成及结构进行鉴定,分析培养基组分和培养温度对该菌株产抑菌脂肽的影响,同时结合电镜观察脂肽对尖孢镰刀菌的抑制作用,为该菌株及其脂肽在农业上的应用奠定一定的基础。
1 材料和方法 1.1 供试材料
1.1.1 供试菌株: 地衣芽胞杆菌Bacillus licheniformis FJAT-4;不同寄主的尖孢镰刀菌致病菌:FJAT-282 (番茄)、FJAT-831 (辣椒)、FJAT-370 (香蕉)、FJAT-9230 (甜瓜)(Fusarium oxysporum)由本实验室分离、保存。
1.1.2 培养基: 本实验所需的培养基组成如表 1所示,其中PDA培养基(新鲜马铃薯20%、葡萄糖2%)用于培养尖孢镰刀菌;K (胰蛋白胨1.0%、酵母膏0.5%、NaCl 0.5%)、L (牛肉膏0.5%、蛋白胨1.0%、酵母膏0.3%、NaCl 0.5%、葡萄糖0.5%)、M (蛋白胨1.0%、酵母膏0.5%、NaCl 1.0%)、O (葡萄糖0.50%、酵母浸膏0.75%、蛋白胨0.75%、硫酸铵0.50%)和P [蛋白胨0.80%、K2HPO4 0.20%,碳源(葡萄糖:蔗糖=1:1) 3.22%、MgSO4 0.05%、酵母膏0.25%]培养基用于培养地衣芽胞杆菌。所用培养基购自北京奥博星生物技术有限公司。
表 1. LC-QTOF-MS/MS测定地衣芽孢杆菌FJAT-4抑菌脂肽组成 Table 1. Lipopeptides produced by Bacillus licheniformis FJAT-4 were identified using LC-QTOF-MS/MS
| RT | MS m/z [M+H]+/[M+Na]+ | MS2 m/z [M+H]+/[M+Na]+ | Identification of lipopeptides |
| 33.397 | 1478.1 | 1102、988、961、944、678 | C17fengycinA |
| 34.844 | 1492.1 | 1116、1002、975、962 | C17fengycin B2 |
| 35.227 | 1506.1 | 1130、1102、1016、988、944 | C17 fengycin B |
| 37.014 | 1462.1 | 1102、988、961 | C16fengycinA derivative |
| 38.138 | 1462.1 | 1102、988、961 | C16fengycinA derivative |
| 39.540 | 1490.1 | 1130、1016、988 | C16fengycinB derivative |
| 42.748 | 1048.8 | 935、822、804、707、661、594、481、391 | C13surfactin derivative |
| 44.782 | 1062.8 | 949、836、818、707、675、594、481、391 | C14surfactin derivative |
| 45.279 | 1062.8 | 949、836、818、707、675、594、481、391 | C14surfactin derivative |
| 46.771 | 1076.9 | 963、850、832、707、689、594、481、391 | C15surfactin derivative |
| 47.449 | 1030.8 | 917、804、707、594、590、481、391 | C13surfactin |
| 49.484 | 1044.8 | 931、818、800、707、594、481、391 | C14surfactin |
| 50.750 | 1058.8 | 945、832、814、707、594、481、391 | C15surfactin |
| 51.383 | 1058.8 | 945、832、814、707、594、481、391 | C15surfactin |
| 51.970 | 1044.8 | 931、818、800、707、594、481、391 | C14surfactin |
表选项
1.2 菌株培养 地衣芽胞杆菌FJAT-4培养:菌株FJAT-4接种至50 mL K液体培养基中,30、170 r/min振荡培养24 h,作为种子液。按1%接种量接种子液至50 mL K/L/M/O/P液体培养基中,相同条件振荡培养48 h。尖孢镰刀菌培养:尖孢镰刀菌接种至50 mL的PDA培养基中,在30、170 r/min下培养2 d后,调整浓度为107 CFU/mL,备用。
1.3 脂肽制备 地衣芽胞杆菌FJAT-4发酵液离心弃去菌体得上清液,上清液加2 mol/L盐酸至pH < 2,4静置24 h后,离心得沉淀,沉淀经低温真空冷冻干燥后为粗脂肽粉末。
1.4 脂肽的LC-QTOF-MS/MS检测
1.4.1 液相色谱条件: 色谱柱为Agilent ZORBAX Extend-C18色谱柱(150 mm×2.1 mm,3.5 ℃m),流速为0.3 mL/min;流动相A为0.1%甲酸水;流动相B为甲醇;洗脱程序:0,60% B;60 min,100% B;65 min,60% B。质谱条件:ESI (+/-)、干燥气温度350 ℃、干燥气流速8 L/min、雾化气压力30 psig、Fragmentor 175 V、Collision Energy 100 V、Skimmer 65 V、扫描方式auto MS/MS;离子扫描范围:100-3000 m/z。
1.4.2 数据处理: 将采集的auto MS/MS数据导入MassHunter工作站软件,进行脂肽化合物一级和二级质谱图提取,结合前体离子、特征碎片离子以及离子裂解规律,并与文献进行比较,推定脂肽结构组成。
1.5 抑菌活性检测 采用抑菌圈法测定地衣芽胞杆菌FJAT-4脂肽对4种不同寄主尖孢镰刀菌的抑制作用。吸取尖孢镰刀菌菌悬液0.5 mL,加入到冷却至50的0.9%琼脂PDA培养基中,菌终浓度为5×105 CFU/mL,混匀后作为上层培养基,倾覆在预先已凝固的1.8%琼脂PDA下层培养基上。待上层培养基凝固,平板冷却后在平板中间打直径7 mm孔,分别注入脂肽粗提物(用甲醇配成终浓度为30、20、10 mg/mL)的溶液80 μL,以无菌甲醇溶液为空白对照,以0.5 mg/mL的潮霉素为阳性对照,每个处理3次重复,30培养10 d后,测抑菌圈直径。
1.6 抑菌脂肽对尖孢镰刀菌菌丝形态结构的影响 从供试的4株尖孢镰刀菌与地衣芽胞杆菌FJAT-4脂肽抑菌圈边缘靠近病原菌一侧切取培养基块,每个处理切取—块,采用2.5%戊二醛进行样品固定。样品经PBS缓冲液、乙醇、叔丁醇处理风干后喷金,利用扫描电镜观察,以未处理正常生长的尖孢镰刀菌病原菌菌丝作为对照。
2 结果和分析 2.1 地衣芽胞杆菌FJAT-4脂肽的结构鉴定 利用LC-QTOF-MS/MS分析地衣芽孢杆菌FJAT-4脂肽结构及组成,结果如表 1所示。保留时间在30-40 min时间段的组分为fengycin类脂肽,保留时间在42-52 min的为surfactin类脂肽。各物质结构鉴定如下。
m/z 1478的碎片离子峰[M+Na]+=1102和988,其[M+H]+=1080和966,丢失的分子量分别是398 Da和512 Da,对应于fengycin A的N-端断裂的脂肪酸-Glu和脂肪酸-Glu-Orn基团,根据相对分子量、特征碎片离子以及离子裂解规律,推测m/z 1478为C17fengycin A,结构如图 1-A所示。准分子离子峰m/z 1462的二级质谱裂解和m/z 1478的相同,说明它们的氨基酸序列是相同的,m/z 1462与fengycin A家族中的m/z 1478离子分子量相差16 Da,差异恰好是含有一个不饱和度的脂肪酸链的“-CH2-”结构,推断为C16fengycin A衍生物。
 |
| 图 1 C17fengycin A (A)、C17Fengycin B (B)和C17fengycin B2 (C)结构图 Figure 1 Cyclic structures of C17fengycin A (A), C17Fengycin B (B) and C17fengycin B2 (C). A: product ions m/z 1478; B: product ions m/z 1506; C: product ions m/z 1492. |
| 图选项 |
m/z 1506的碎片离子峰[M+Na]+ m/z=1130和1016,其[M+H]+ m/z=1108和994,丢失的分子量分别是398 Da和512 Da,对应于fengycin B的N-端断裂的脂肪酸-Glu和脂肪酸-Glu-Orn基团,其与m/z 1478的分子量相差28 Da,说明它们的肽链上氨基酸为Ala或Val,根据相对分子量、特征碎片离子以及离子裂解规律,推断m/z 1506为C17 fengycin B,结构如图 1-B所示。准分子离子峰m/z 1490的二级质谱裂解和m/z 1506的相同,说明它们的氨基酸序列是相同的,m/z 1490与fengycin B家族中的m/z 1506的离子分子量相差16 Da,差异恰好是含有1个不饱和度的脂肪酸链的“-CH2-”结构,推断为C16fengycin B衍生物。
m/z 1492的碎片离子峰[M+Na]+=1116和1002,其[M+H]+=1094和980,丢失的分子量分别是398 Da和512 Da,对应于fengycin B的N-端断裂的脂肪酸-Glu和脂肪酸-Glu-Orn基团,其与m/z 1506的分子量相差14 Da,说明它们的肽链上氨基酸为Ile或Val,根据相对分子量、特征碎片离子以及离子裂解规律,推测m/z1492为C17 fengycin B2,结构如图 1-C所示。
m/z 1030的产物离子为917、804、707、594、590、481。其中917、804和590为含脂肪酸链的N-端产物离子,是m/z 1030分别丢失Leu、Leu-Leu和Leu-Leu-Asp-Val基团后的产物离子;707、594和481是C-端肽段产物离子,是m/z 1030分别丢失C13 β-羟基脂肪酸链-Glu、C13 β-羟基脂肪酸链-Glu-Leu和C13 β-羟基脂肪酸链-Glu-Leu-Leu基团后的产物离子,推断m/z 1030为C13surfactin,结构如图 2所示。m/z 1044、m/z 1058的C-端肽段产物离子(707、594、481)和m/z1030的一致,说明了它们的氨基酸序列是相同的。m/z1044、m/z 1058的N-端产物离子与m/z 1030的N-端产物离子分子量相差14 Da,差异是侧链-CH2基团,推断m/z1044和m/z 1058为C14surfactin和C15surfactin。
 |
| 图 2 C13surfactin结构图 Figure 2 Cyclic structures of C13surfactin. Product ions m/z 1030. |
| 图选项 |
[M+Na]+分子量为1048.8、1062.8和1076.9的C-端肽段产物离子(707、594、481)和surfactin的m/z1030、1044和1058的相同,说明它们的氨基酸序列是相同的。m/z 1048、1062和1076.9的N-端产物离子分子量相差14 Da,差异是侧链-CH2基团。m/z1048与m/z1044的N-端产物离子分子量相差4,恰好是C与O的分子量差异,推测m/z 1048、1062和1076为C13-C15surfactin衍生物,同结构的这一系列分子量的化合物未见文献报道。
2.2 培养基成分对地衣芽胞杆菌FJAT-4脂肽组成和抑菌作用影响 根据文献报道[22-25],选择K、L、M、O和P五种不同培养基进行地衣芽胞杆菌FJAT-4培养,结果表明采用这5种培养基,菌株FJAT-4均能够代谢产生脂肽。利用液相色谱串联四极杆飞行时间质谱(LC-QTOF-MS/MS)测定了不同培养基培养的地衣芽胞杆菌FJAT-4脂肽组成,其总离子流图如图 3所示。各培养基产生的脂肽均由fengycin和surfactin组成,其相对含量分别是:63.6%和36.4% (K)、62.1%和37.9% (L、30oC)、56.3%和43.7% (M)、66.2%和33.8% (O)以及69.1%和30.9% (P),上述结果表明培养基组分对脂肽组成影响较小。
 |
| 图 3 不同培养基的地衣芽胞杆菌FJAT-4脂肽组成总离子流图 Figure 3 The total ion flow spectra of lipopeptides separated from Bacillus licheniformis FJAT-4 under different culture medium. |
| 图选项 |
抑菌实验结果表明,5种培养基培养产生的脂肽均能够有效抑制辣椒尖孢镰刀菌FJAT-831的生长,抑菌圈直径范围分别是:(18.36±0.27) mm (K)、(24.51±0.40)mm (L)、(22.70±0.60) mm (M)、(21.41±0.45) mm (O)及(20.16±0.73)mm (P),强于阳性对照0.5 mg/mL的潮霉素[(17.36±0.42) mm],其中采用L培养基培养得到的脂肽抑菌效果最强。
2.3 培养温度对地衣芽胞杆菌FJAT-4脂肽组成和抑菌作用影响 选择L培养基,在20、25、30、35、40 ℃进行培养地衣芽胞杆菌FJAT-4发酵培养,结果表明菌株在20 ℃和25 ℃下培养,菌株FJAT-4不产生脂肽;30-40 ℃培养,菌株FJAT-4能够产生脂肽,其总离子流图如图 4所示。培养温度为30 ℃和35 ℃时,脂肽组成为fengycin (30 ℃:62.1%;35 ℃:59.3%)和surfactin (30 ℃:37.9%;35 ℃:40.7%);培养温度为40 ℃时,脂肽组成以surfactin为主(79.7%),fengycin的比例低(20.3%)。上述结果表明,温度对地衣芽胞杆菌FJAT-4代谢产生脂肽影响很大。
 |
| 图 4 不同温度培养的地衣芽胞杆菌FJAT-4脂肽组成总离子流图 Figure 4 The total ion flow spectra of lipopeptides separated from Bacillus licheniformis FJAT-4 under different culture temperature. |
| 图选项 |
抑菌实验结果表明,30-40 ℃培养产生的脂肽均能够有效抑制辣椒尖孢镰刀菌FJAT-831的生长,在培养初期,30 ℃和35 ℃发酵产生的脂肽效果强于40 ℃;在培养后期,40 ℃发酵产生的脂肽抑制辣椒尖孢镰刀菌FJAT-831效果最好,这归因于surfactin的协同增效作用,当surfactin含量达到一定程度时,能有效增强fengycin抑制真菌的能力。
2.4 地衣芽胞杆菌FJAT-4脂肽浓度对抑菌作用影响研究 采用L培养基培养FJAT-4,研究了不同浓度脂肽对辣椒尖孢镰刀FJAT-831、番茄尖孢镰刀FJAT-282、香蕉尖孢镰刀菌FJAT-370、甜瓜尖孢镰刀菌FJAT-9230的抑制效果(表 2)。结果表明地衣芽胞杆菌FJAT-4产生的脂肽对这四种作物尖孢镰刀菌病原菌均具有较强的抑制效果,且脂肽浓度越高,抑制病原真菌效果越明显,呈现剂量效应。
表 2. 地衣芽胞杆菌FJAT-4脂肽对尖孢镰刀菌的抑菌活性 Table 2. Anfungal activities of lipopeptides produced by Bacillus licheniformis FJAT-4 against Fusarium oxysporum
| Indicator strains | Diameter of inhibition zone/mm | ||||
| FJAT-831 | FJAT-282 | FJAT-370 | FJAT-9230 | ||
| Concentration of crude lipopeptides /(mg/mL) | 10 | 11.06±0.60c | 14.55±0.96b | 16.52±0.99a | 13.03±2.29b |
| 20 | 18.48±0.92b | 17.61±1.58a | 17.71±0.73a | 17.37±1.01ab | |
| 30 | 24.51±0.42a | 19.11±0.48a | 18.38±1.71a | 19.51±0.59a | |
| The difference letter in the same column indicated that the difference between the grades is significantly through Duncan test (P < 0.05). | |||||
表选项
2.5 地衣芽胞杆菌FJAT-4菌脂肽对尖孢镰刀菌丝形态的影响 利用电镜观察正常尖孢镰刀菌菌丝、孢子和抑菌圈边缘的尖孢镰刀菌菌丝和孢子形态,结果如图 5所示。图A1中正常的尖孢镰刀菌菌丝边缘光滑、粗细均匀,图A2中抑菌圈边缘的菌丝断裂,孢子变成球形、且表面有乳突状的物质,有些孢子已经塌陷;图B1、C1、D1中正常的尖孢镰刀菌菌丝通体饱满、粗细均匀,可见产孢细胞,图B2、C2、D2中抑菌圈边缘的尖孢镰刀菌菌丝体断裂、表面皱缩,凹凸不平,未见孢子。上述结果表明抑菌脂肽会致使菌丝体发育畸形,影响尖孢镰刀菌的正常生长。
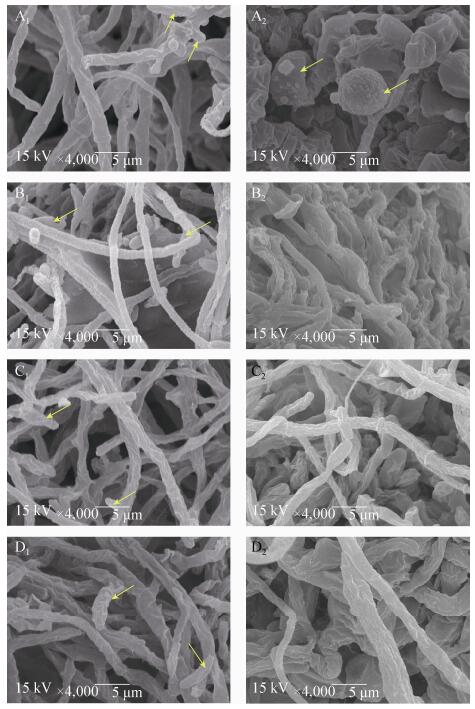 |
| 图 5 尖孢镰刀菌菌丝形态的扫描电镜观察 Figure 5 Mycelial morphology of F. oxysporum under scanning electron microscope. A1: normal mycelial and spore of F. oxysporum FJAT-831; A2: mycelial and spore of F. oxysporum FJAT-831 along the zone of inhibition; B1: normal mycelial of F. oxysporum FJAT-282; B2: mycelial of F. oxysporum FJAT-282 along the zone of inhibition; C1: normal mycelial of F. oxysporum FJAT-370; C2: mycelial of F. oxysporum FJAT-370 along the zone of inhibition; D1: normal mycelial of F. oxysporum FJAT-9230; D2: mycelial of F. oxysporum FJAT-9230 along the zone of inhibition. Arrows means sporulation cells and spores. Magnification: × 4000. |
| 图选项 |
3 讨论 地衣芽胞杆菌对植物的真菌性病害有较好的抑制效果,如烟草黑胫病、番茄灰霉病、棉花枯萎和黄萎病等,在作物病害防治方面得到广泛的应用[26]。葛慈斌等[21]研究表明地衣芽胞杆菌FJAT-4能够有效抑制尖孢镰刀菌的生长,但其抑菌活性物质还不明确。作者前期研究发现地衣芽胞杆菌FJAT-4所分泌的脂肽类物质对镰刀菌具有很好的抑制效果,因而本文进一步利用LC-QTOF-MS/MS技术对该抑菌脂肽组成及结构进行鉴定,分析培养基组分和培养温度对该菌株产抑菌脂肽的影响,并结合扫描电镜观察该脂肽对尖孢镰刀菌菌丝体的抑制作用,为脂肽和菌株的进一步利用奠定基础。
地衣芽胞杆菌能够产生地衣素、plipastatin、surfactin、fengycin等多种脂肽类化合物,不同菌株产生的脂肽组成不一样。Tendulkar等[27]从地衣芽胞杆菌BC98中分离出对稻瘟病原菌具有较强抑制效果的脂肽类抗生素surfactin (分子量1035 Da);Pecci等[6]从地衣芽胞杆菌V9T14代谢物中检测到多种脂肽类化合物,包括C13-C15 surfactin同系物、C17 fengycin A、C17 fengycin B及C14-C16 fengycin同系物,其对多种人类致病菌具有较强的抗性。Joshi等[28]研究发现地衣芽胞杆菌W16产生的类地衣素A脂肽能够提高原油采收率。本研究结果表明地衣芽胞杆菌FJAT-4分泌产生的抑菌脂肽对多种寄主的尖孢镰刀病原菌均有显著的抑制效果,且呈浓度依赖性,该脂肽是由C17fengycin A、C17fengycin B、C17fengycin B2、C16fengycin A衍生物、C16fengycin B衍生物、C13-C15surfactin及C13-C15surfactin衍生物组成,其中C13-C15surfactin (m/z[M+Na]+=1048.6/1062.6/1076.6)衍生物为新化合物,未见国内外文献报道。
芽胞杆菌代谢产生的脂肽种类与培养基成分和培养条件有关,Nihorimbere等[24]发现脂肪酸盐(如柠檬酸盐和苹果酸盐)有利于surfactin合成,而糖类物质(如葡萄糖、果糖和麦芽糖)则有利于iturin和fengycin积累;赵朋超等[23]发现葡萄糖有利于fengycin的产生,而甘油和山梨醇对bacillomycin D合成有促进作用;Yang等[25]研究表明菌株在不同生长时间产生的脂肽不同,稳定生长期的早期产生fengycin,在指数生长期向稳定生长期过渡的过程中产生大量surfactin,在更加靠后的时间段内产生Iturin。Ohno等[29]研究发现枯草芽孢杆菌RB14的培养温度为25 ℃时,iturin的产量最大,而30 ℃是产生surfactin的最适温度。而本研究中五种培养基培养的地衣芽孢杆菌FJAT-4代谢产生的脂肽均以fengycin为主(55%-70%);该菌株在低温时(20-25 ℃)不能代谢产生脂肽物质,30 ℃和35 ℃时菌株FJAT-4代谢产生的surfactin比例分别是37.9%和40.7%,而当培养温度更高时(40 ℃)产生的脂肽中surfactin比例更高,达80%,说明温度会强烈影响地衣芽胞杆菌FJAT-4抑菌脂肽的产生,并且高温有利于提高脂肽中surfactin的比例。
Fengycin是芽胞杆菌产生的一类对植物病原真菌有较强的拮抗作用的抑菌脂肽,研究表明脂肽之间存在协同增效的作用,surfactin和fengycin以及iturin和fengycin的混合使用都可以很大程度上提高单一脂肽的生物活性[18]。本研究发现40 ℃培养产生的脂肽中surfactin约占脂肽总量的80%,而其他温度脂肽中surfactin约占脂肽总量的30%-45%,该温度下产生的脂肽混合物抑菌效果强于其他温度,说明了surfactin能够有效增强fengycin抑制真菌的能力,进一步证实了surfactin与fengycin之间的协同增效作用,尚未见同类研究报道。
Fengycin的抑菌机制可能是改变病原菌细胞膜通透性(低浓度)或作用于生物膜以破坏病菌细胞膜结构(高浓度)[30]。胡陈云等[31]研究发现枯草芽胞杆菌ge25产生的fengycin类脂肽(含C16 fengycin A和C15-C18 fengycin B同系物或衍生物)会显著抑制人参黑斑菌和人参锈腐菌的正常生长,会破坏病原菌的菌丝结构,改变菌丝细胞膜通透性。本文通过扫描电镜观察也发现,被脂肽抑制的尖孢镰刀菌菌丝断裂变形,孢子变形或显著抑制了孢子的生长,表明地衣芽胞杆菌FJAT-4产生的抑菌脂肽会致使尖孢镰刀菌菌丝体发育畸形,影响尖孢镰刀菌的正常生长。本研究明确了地衣芽胞杆菌发酵生产的脂肽对多种尖孢镰刀菌的抑制作用,这为菌株的后续研究及在枯萎病防治中的推广应用奠定了良好基础。
References
| [1] | Chowdhury SP, Uhl J, Grosch R, Alquéres S, Pittroff S, Dietel K, Schmitt-Kopplin P, Borriss R, Hartmann A. Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Molecular Plant-Microbe Interactions, 2015, 28(9): 984-995. DOI:10.1094/MPMI-03-15-0066-R |
| [2] | Rocha DJA, Moura AB. Biological control of tomato wilt caused by Ralstonia solanacearum and Fusarium oxysporum f. sp. lycopersici by rhizobacteria. Tropical Plant Patholog, 2013, 38(5): 423-430. DOI:10.1590/S1982-56762013005000025 |
| [3] | Etchegaray A, de Castro Bueno C, de Melo IS, Tsai SM, Fiore MF, Silva-Stenico ME, de Moraes LAB, Teschke O. Effect of a highly concentrated lipopeptide extract of Bacillus subtilis on fungal and bacterial cells. Archives of Microbiology, 2008, 190(6): 611-622. DOI:10.1007/s00203-008-0409-z |
| [4] | Berrue F, Ibrahim A, Boland P, Kerr RG. Newly isolated marine Bacillus pumilus (SP21): A source of novel lipoamides and other antimicrobial agents. Pure and Applied Chemistry, 2009, 81(6): 1027-1031. |
| [5] | Roy A, Mahata D, Paul D, Korpole S, Franco OL, Mandal SM. Purification, biochemical characterization and self-assembled structure of a fengycin-like antifungal peptide from Bacillus thuringiensis strain SM1. Frontiers in Microbiology, 2013, 4: 332. |
| [6] | Pecci Y, Rivardo F, Martinotti MG, Allegrone G. LC/ESI-MS/MS characterisation of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. Journal of Mass Spectrometry, 2010, 45(7): 772-778. DOI:10.1002/jms.v45:7 |
| [7] | Zhuang GH. Screening GD stain producing antimicrobial lipopeptide and studying on its separation, identification and application. Doctoral Dissertation of Yangzhou University, 2014. (in Chinese) 庄国宏. 产抗菌脂肽GD菌株筛选及脂肽分离、鉴定与应用研究. 扬州大学博士学位论文. 2014. http://cdmd.cnki.com.cn/Article/CDMD-11117-1014355882.htm |
| [8] | Xiong HQ, Cai YF, Guo ZZ, Zhang YY, Cao Y. Advances of lipopeptides in Bacillus. Hubei Agricultural Sciences, 2015, 54(12): 2817-2821. (in Chinese) 熊汉琴, 蔡燕飞, 郭真真, 张娅娅, 曹禺. 芽孢杆菌脂肽类抗生素的研究进展. 湖北农业科学, 2015, 54(12): 2817-2821. |
| [9] | Grau A, Ortiz A, de Godos A, Gómez-Fernández JC. A biophysical study of the interaction of the lipopeptide antibiotic iturin A with aqueous phospholipid bilayers. Archives of Biochemistry and Biophysics, 2000, 377(2): 315-323. DOI:10.1006/abbi.2000.1791 |
| [10] | Kuo CH, Lin YW, Chen RS. Lipopeptides extract from Bacillus amyloliquefaciens induce human oral squamous cancer cell death. Asian Pacific Journal of Cancer Prevention, 2015, 16(1): 91-96. DOI:10.7314/APJCP.2015.16.1.91 |
| [11] | Li JF, Liu L. Research progress of lipopeptide biosurfactant. Chemistry & Bioengineering, 2015, 32(1): 12-15. (in Chinese) 李俊峰, 刘丽. 脂肽类生物表面活性剂的研究进展. 化学与生物工程, 2015, 32(1): 12-15. |
| [12] | Arrebola E, Jacobs R, Korsten L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. Journal of Applied Microbiology, 2010, 108(2): 386-395. DOI:10.1111/jam.2009.108.issue-2 |
| [13] | Nerurkar AS. Structural and molecular characteristics of lichenysin and its relationship with surface activity//. Biosurfactants. New York: Springer, 2010: 304-315. http://www.ncbi.nlm.nih.gov/pubmed/20545292 |
| [14] | Pathak KV, Bose A, Keharia H. Identification and characterization of novel surfactins produced by fungal antagonist Bacillus amyloliquefaciens 6B. Biotechnology and Applied Biochemistry, 2014, 61(3): 349-356. |
| [15] | Heerklotz H. Interactions of surfactants with lipid membranes. Quarterly Reviews of Biophysics, 2008, 41(3/4): 205-264. |
| [16] | Fehri LF, Wróblewski H, Blanchard A. Activities of antimicrobial peptides and synergy with enrofloxacin against Mycoplasma pulmonis. Antimicrobial Agents and Chemotherapy, 2007, 51(2): 468-474. DOI:10.1128/AAC.01030-06 |
| [17] | Deleu M, Paquot M, Nylander T. Fengycin interaction with lipid monolayers at the air-aqueous interface—implications for the effect of fengycin on biological membranes. Journal of Colloid and Interface Science, 2005, 283(2): 358-365. DOI:10.1016/j.jcis.2004.09.036 |
| [18] | Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny JL, Thonart P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environmental Microbiology, 2007, 9(4): 1084-1090. DOI:10.1111/emi.2007.9.issue-4 |
| [19] | Wise F, Falardeau J, Hagberg I, Avis TJ. Cellular lipid composition affects sensitivity of plant pathogens to fengycin, an antifungal compound produced by Bacillus subtilis strain CU12. Phytopathology, 2014, 104(10): 1036-1041. DOI:10.1094/PHYTO-12-13-0336-R |
| [20] | Zheng XF, Ge CB, Lin YZ, Liu J, Liu B. Identification on two biocontrol Bacillus strains BS-2000 and JK-2 from fusarium wilt disease of melon crop. Fujian Journal of Agricultural Sciences, 2006, 21(2): 154-157. (in Chinese) 郑雪芳, 葛慈斌, 林营志, 刘建, 刘波. 瓜类作物枯萎病生防菌BS-2000和JK-2的分子鉴定. 福建农业学报, 2006, 21(2): 154-157. |
| [21] | Ge CB, Liu B, Xiao RF, Zhu YJ, Tang JY. Effect of temperature on the growth and antifungal activity of Fusarium-wilt-disease biocontrol bacterium strain FJAT-4. Fujian Journal of Agricultural Sciences, 2013, 28(7): 697-704. (in Chinese) 葛慈斌, 刘波, 肖荣凤, 朱育菁, 唐建阳. 枯萎病生防菌FJAT-4的生长与抑菌作用的温度效应. 福建农业学报, 2013, 28(7): 697-704. |
| [22] | Wei YH, Lai CC, Chang JS. Using Taguchi experimental design methods to optimize trace element composition for enhanced surfactin production by Bacillus subtilis ATCC 21332. Process Biochemistry, 2007, 42(1): 40-45. DOI:10.1016/j.procbio.2006.07.025 |
| [23] | Zhao PC, Quan CS, Jin LM, Wang LN, Fan SD. Effects of different nitrogen and carbon sources on the production of antifungal lipopeptides from Bacillus amyloliquefaciens Q-426. China Biotechnology, 2012, 32(10): 50-56. (in Chinese) 赵朋超, 权春善, 金黎明, 王丽娜, 范圣第. 氮源和碳源对解淀粉芽孢杆菌Q-426抗菌脂肽合成的影响. 中国生物工程杂志, 2012, 32(10): 50-56. |
| [24] | Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiology Ecology, 2012, 79(1): 176-191. DOI:10.1111/j.1574-6941.2011.01208.x |
| [25] | Yang CK, Tai PC, Lu CD. Time-related transcriptome analysis of B. Subtilis 168 during growth with glucose. Current Microbiology, 2014, 68(1): 12-20. DOI:10.1007/s00284-013-0432-4 |
| [26] | Yang Y, Zhang FY, Cang GL, Wang B, Lu H. Research advances in the application of bioactive substances produced by Bacillus licheniformis. Current Biotechnology, 2013, 3(1): 22-26. (in Chinese) 杨阳, 张付云, 苍桂璐, 王斌, 卢航. 地衣芽胞杆菌生物活性物质应用研究进展. 生物技术进展, 2013, 3(1): 22-26. |
| [27] | Tendulkar SR, Saikumari YK, Patel V, Raghotama S, Munshi TK, Balaram P, Chattoo BB. Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. Journal of Applied Microbiology, 2007, 103(6): 2331-2339. DOI:10.1111/jam.2007.103.issue-6 |
| [28] | Joshi SJ, Al-Wahaibi YM, Al-Bahry SN, Elshafie AE, Al-Bemani AS, Al-Bahri A, Al-Mandhari MS. Production, characterization, and application of Bacillus licheniformis W16 biosurfactant in enhancing oil recovery. Frontiers in Microbiology, 2016, 7: 1853. |
| [29] | Ohno A, Ano T, Shoda M. Effect of temperature on production of lipopeptide antibiotics, iturin A and surfactin by a dual producer, Bacillus subtilis RB14, in Solid-State fermentation. Journal of Fermentation and Bioengineering, 1995, 80(5): 517-519. DOI:10.1016/0922-338X(96)80930-5 |
| [30] | Guo QG, Dong WX, Li SZ, Lu XY, Wang PP, Zhang XY, Wang Y, Ma P. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiological Research, 2014, 169(7/8): 533-540. |
| [31] | Hu CY, Li Y, Liu M, Qin MJ, Ding WL. Antagonism of Bacillus subtilis ge25 against two kinds of ginseng pathogens and identification of antifungal lipopeptide metabolites. Chinese Journal of Biological Control, 2015, 31(3): 386-393. (in Chinese) 胡陈云, 李勇, 刘敏, 秦民坚, 丁万隆. 枯草芽孢杆菌ge25对两种人参病原菌的抑制作用及脂肽类抑菌代谢产物的鉴定. 中国生物防治学报, 2015, 31(3): 386-393. |
