范青云, 张舒梦, 巩玉静, 何进


华中农业大学生命科学技术学院, 农业微生物学国家重点实验室, 湖北 武汉 430070
收稿日期:2016-05-19;修回日期:2016-06-29;网络出版日期:2016-07-19
基金项目:国家自然科学基金(31270105)
*通信作者:何进, Tel/Fax:+86-27-87280670;E-mail:hejin@mail.hzau.edu.cn
摘要: [目的]探究双组份系统YvcPQ影响芽胞形成的机制。[方法]利用β-半乳糖苷酶活性实验验证YvcP对芽胞形成抑制因子KapD的调控作用;通过无痕基因敲除并分别比较突变株与出发菌株的芽胞产率,研究YvcPQ及KapD对芽胞形成的影响;应用细菌单杂交实验、EMSA实验和实时荧光定量PCR技术探究转录调控因子AbrB对yvcPQ操纵子的转录调节。[结果]YvcP可以正调控kapD的表达,从而抑制芽胞形成;yvcPQ不能受YvcP的自调控,而是受AbrB转录激活。[结论]调控因子AbrB能够通过正调控yvcPQ操纵子的转录来提高细胞内YvcP的含量,进而增强YvcP对芽胞形成抑制因子编码基因kapD的表达,最终抑制芽胞的形成。
关键词: 苏云金芽胞杆菌 双组份系统 YvcPQ AbrB 芽胞形成
Regulation of sporulation by two-component system YvcPQ in Bacillus thuringiensis
Fan Qingyun, Zhang Shumeng, Gong Yujing, He Jin


State Key Laboratory of Agricultural Microbiology, College of Life Science and Technology, Huazhong Agricultural University, Wuhan 430070, Hubei Province, China
Received 19 May 2016; Revised 29 June 2016; Published online 19 July 2016
*Corresponding author: Jin He, Tel/Fax:+86-27-87280670;E-mail:hejin@mail.hzau.edu.cn
Supported by the National Natural Science Foundation of China (31270105)
Abstract: [Objective]To study the regulation of sporulation controlled by two-component system (TCS) YvcPQ.[Methods]β-galactosidase experiment was used to verify the regulation of YvcP on kapD expression; bacterial one-hybrid assay, EMSA and RT-qPCR were applied to study the regulation of AbrB on yvcPQ expression; markerless gene deletion coupled with spore count was used to reveal the influence of yvcPQ and kapD expressions on sporulation.[Results]The transcriptional regulator AbrB up-regulated the expression of yvcPQ; YvcP promoted the expression of kapD to inhibit sporulation.[Conclusion]AbrB up-regulated the transcription of yvcPQ operon, then the increased YvcP strengthened the transcriptional acitivation of sporulation inhibitor gene kapD, and subsequently inhibited sporulation.
Key words: Bacillus thuringiensis two-component system YvcPQ AbrB sporulation
双组份系统(Two-component system,TCS)是存在于微生物细胞中的信号转导系统,由位于细胞膜上的组氨酸激酶(Histidine kinase,HK)和位于细胞质中的响应调节子(Response regulator,RR)组成[1-2]。HK能够感应环境中或细胞内的某些信号的刺激,并发生自磷酸化,自磷酸化的HK将磷酸基团传递给其对应的RR,使RR磷酸化;被磷酸化的RR作为转录因子调控相关基因的表达,从而对信号产生反应[3]。TCS响应的信号刺激很广泛,其调控的下游基因也多种多样,涉及到细菌的生长、芽胞形成、渗透压、抗生素抗性、趋化性、毒性、致病性以及营养物质的代谢等各个方面。
YvcPQ是芽胞杆菌中普遍存在的TCS,由YvcQ (HK)和YvcP (RR)组成。目前关于YvcPQ的生物学功能及其调控机制的报导很少,且主要集中在Bacillus subtilis中。2003年,Mascher等最早在B. subtilis中发现YvcPQ可以通过调控位于yvcPQ下游的yvcRS操纵子的转录来响应杆菌肽刺激[4]。随后的研究表明,YvcPQ与另一响应杆菌肽的TCS BceRS存在信号交叉[5-6]。2011年的研究将YvcPQ的响应范围扩大到其他一些羊毛硫类抗生素[7]。本实验室通过生物信息学分析发现在B. thuringiensis BMB171中YvcP (编码基因BMB171_C4102,新编号BMB171_RS22135)调控4个候选基因:yvcR (BMB171_C4100,新编号BMB171_RS22125)、BMB171_C4385 (新编号BMB171_RS23635)、kapD (BMB171_C4525,新编号BMB171_RS24515)和BMB171_C4835 (新编号BMB171_RS26135),利用细菌单杂交实验及实时荧光定量PCR进行了验证,并进一步证实了YvcP能够转录激活yvcR和yvcS1S2,从而对杆菌肽产生抗性[8]。
kapD在基因组上被注释为能够编码芽胞形成抑制因子KapD的基因,KapD能够抑制芽胞形成过程中的KinA途径[9]。AbrB为调控因子,能够阻遏与芽胞和生物膜形成相关基因的转录。本研究以B. thuringiensis BMB171为研究对象,发现调控因子AbrB (编码基因BMB171_C0031,新编号BMB171_ RS00200)激活yvcPQ的转录,而YvcP能够通过正调控芽胞形成抑制因子KapD编码基因的表达,从而抑制芽胞的形成。由于蜡样芽胞杆菌群菌株之间基因组极其相似,本研究结果为探究蜡样芽胞杆菌群中YvcPQ与芽胞形成的关系,全面揭示芽胞形成的调控机制奠定了良好的基础。
1 材料和方法 1.1 菌株与质粒 本研究所用菌株与质粒见表 1。
表 1. 本研究所用的菌株和质粒 Table 1. Strains and plasmids used in this study
| Strains and Plasmids | Characteristics | Source |
| Strains | ||
| E. coli DH5α | A cloning host | Laboratory stock |
| E. coli XR | A recipient strain of E. coli for bacterial one-hybrid system | [10] |
| BMB171 | An acrystalliferous B. thuringiensis strain | [11] |
| ΔyvcPQ | yvcPQ markerless deletion mutant derived from BMB171 | [8] |
| ΔkapD | kapD markerless deletion mutant derived from BMB171 | [8] |
| ΔabrB | abrB markerless deletion mutant derived from BMB171 | This study |
| Rv2031p/Rv3133c-hybrid | E. coli XR containing pBX-Rv2031p and pTRG-Rv3133c, positive control | [10] |
| pTRG/pBX-PyvcPQ | E. coli XR containing plasmids pTRG and pBX-PyvcPQ, self-activated control | This study |
| pTRG-yvcP/pBX | E. coli XR containing plasmids pTRG-yvcP and pBX-PyvcS1S2, self-activated control | This study |
| pTRG-yvcP/pBX-PyvcPQ | E. coli XR containing plasmids pTRG-yvcP and pBX-PyvcPQ | This study |
| pTRG/pBX | E. coli XR containing plasmids pTRG and pBX, negative control | This study |
| BMB171-pHT1K-PkapD-lacZ | BMB171 containing the plasmid pHT1K-PkapD-lacZ | This study |
| ΔyvcPQ-pHT1K-PkapD-lacZ | ΔyvcPQ containing the plasmid pHT1K-PkapD-lacZ | This study |
| BMB171-pHT1K-lacZ | BMB171 containing the plasmid pHT1K-lacZ | This study |
| ΔyvcPQ-pHT1K-lacZ | ΔyvcPQ containing the plasmid pHT1K-lacZ | This study |
| BL21-pET-abrB | BL21(DE3) containing the overexpression plasmid pET28b-abrB | This study |
| DH5α-pRP1028 | E. coli DH5α containing the plasmid pRP1028 | [12] |
| DH5α-pSS4332 | E. coli DH5α containing the plasmid pSS4332 | [12] |
| DH5α-pSS1827 | E. coli DH5α containing the plasmid pSS1827 | [12] |
| Plasmids | ||
| pRP1028 | Spc, shuttle plasmid, with temperature-sensitive suicide B. thuringiensis replicon, Ⅰ-Sce Ⅰ cleavage site and ori T, etc. | [12] |
| pSS4332 | Kan, expression plasmid for expressing Ⅰ-Sce Ⅰ restriction enzyme | [12] |
| pSS1827 | Amp, helper plasmid for conjugation during gene deletion | [12] |
| pRP1028-UabrB-DabrB | pRP1028 containing upstream and downstream homologous arms of abrB (UabrB and DabrB) | This study |
| pTRG | Tet, ColE1 replicon, lpp/lac-UV5 promoter, used for bacterial one-hybrid assays | [10] |
| pBX | Chl, p15A replicon, lac-UV5 promoter, used for bacterial one-hybrid assays | [10] |
| pTRG-yvcP | yvcP in BamH Ⅰ and Xho Ⅰ sites of pTRG | This study |
| pBX-PyvcPQ | The promoter region of yvcPQ in Xcm Ⅰ site of pBX | This study |
| pHT1K-lacZ | Amp, Ery, E. coli-B. thuringiensis shuttle vector containing lacZ gene | Laboratory stock |
| pHT1K-PkapD-lacZ | pHT1K carrying kapD promoter region fused with lacZ gene | This study |
表选项
1.2 引物 本研究所用引物见表 2。
表 2. 本研究所使用的引物 Table 2. Primers used in this study
| Primers | Sequences (5′→3′) |
| yvcP F | CTGAGGATCCATGGAAAAAGGAGTAAATGTAAAAG |
| yvcP R | TTACAGTTTTGTCATTTTTCTCATC |
| PkapD F | CTAACCATGGTGCGGATGTAAATGAAAG |
| PkapD R | GCGGATCCACGATCCCTTCCTTAT |
| PyvcPQ F | TTACACGTGAAAGTGATGGC |
| PyvcPQ R | TGTTCGTTTTCTCCCCATAA |
| QyvcPQ F | GAGTGGGGCGAAGGGCAT |
| QyvcPQ R | AGTAAAGAGTCATCTAATGTAGTTCCACC |
| Q16S rRNA F | TTTTGCTAGCGCTTTCGCAG |
| Q16S rRNA R | TAGCGCCTGTTGTGAAGGTG |
表选项
1.3 试剂与耗材 各类限制性内切酶,Taq DNA聚合酶,DNA Ladder Marker,pMD19-T simple vector,质粒抽提试剂盒,PrimeScriptTM RT reagent Kit with gDNA Eraser来自TaKaRa Bio Inc;T4 DNA连接酶来自Thermo Fisher Scientific Inc;细菌总DNA抽提试剂盒、琼脂糖凝胶DNA回收试剂盒来自Bioteke公司;壮观霉素(Spc)、硫酸多粘菌素(Pmx)、氨苄青霉素(Amp)、硫酸卡那霉素(Kan)、红霉素(Ery)、氯霉素(Chl)、四环素(Tet)和链霉素(Str)来自美国Sigma公司;TRIzol Reagent来自Life Technoligies Corporation;荧光定量PCR试剂盒来自康为世纪生物科技有限公司。
常用化学药品来自国药集团化学试剂有限公司。引物合成和DNA测序由武汉天一辉远生物科技有限公司完成。
1.4 β-半乳糖苷酶实验 具体的实验操作及计算方法参考文献[13]。
1.5 芽胞计数 分别挑取单菌落于5 mL无抗性LB培养基中28 ℃、200 r/min振荡培养12 h,然后分别以1:100的接种量转接于5 mL GYS培养基中,振荡培养9 h,之后转接入200 mL GYS培养基中,保证各菌液的起始OD600为0.01,28 ℃、200 r/min振荡培养至芽胞期。分别取1 mL菌液在70 ℃中水浴处理1 h,然后将OD600调一致,梯度稀释,涂布无抗性的GYS固体平板,28 ℃培养12 h后,记录各平板上的菌落形成单位(CFU)[14]。每次实验3个生物学重复。
1.6 细菌单杂交实验 方法参照文献[8, 10, 15]。具体操作如下:首先以BMB171的基因组DNA为模板,yvcP F/yvcP R为引物(表 2),PCR扩增目的蛋白基因yvcP。将扩增的片段用BamHⅠ和XhoⅠ双酶切后,与同样双酶切的pTRG载体连接获得表达载体pTRG-yvcP (YvcP与RNA聚合酶的α亚基融合表达)。以BMB171的基因组DNA为模板,PyvcPQ F/PyvcPQ R为引物(表 2),扩增yvcPQ操纵子上游349 bp的DNA片段,为yvcPQ的启动子区域,用PyvcPQ表示。用Taq DNA聚合酶扩增时会在DNA片段的末端加上1个碱基A;pBXcmT载体上有2个相邻的XcmⅠ酶切位点,经过XcmⅠ特异性酶切后会在切口两端分别留下T末端。因此,可以直接以TA克隆的方式将PyvcPQ连接到pBXcmT载体报告基因His3-aadA (使共转化菌株能够在含有抑制组氨酸合成的3AT及Str的M9培养基上生长)的上游,得到报告载体pBX-PyvcPQ。
接着,将表达载体pTRG-yvcP与报告载体pBX-PyvcPQ共转化到宿主菌株E. coli XR中得到待验证的共转化菌株pTRG-yvcP/pBX-PyvcPQ;将载体pTRG与pBX-PyvcPQ共转化到E. coli XR中得到片段特异性自激活负对照菌株pTRG/pBX-PyvcPQ;将载体pTRG-yvcP与pBX共转化到E. coli XR中得到蛋白特异性自激活负对照菌株pTRG-yvcP/pBX;含有Rv3133c的pTRG载体和含有Rv2031p的pBX载体的共转化菌株Rv2031p/Rv3133c-hybrid为正对照;pTRG/pBX为负对照(表 1)。
最后,将各个共转化菌株依次点滴在不含有3AT (3-氨基-1, 2, 4-三氮唑)和Str以及含有3AT和Str的M9培养基固体平板上,30 ℃培养24 h,观察菌株的生长情况。
1.7 EMSA实验 参照文献[16],具体操作如下:FAM标记的目的DNA PyvcPQ和AbrB蛋白质(由菌株BL21-pET-abrB过表达获得)于室温孵育0.5 h。孵育体系一般为20 μL,其中DNA为8 μL,外加2 μL的10×H缓冲液,蛋白质的体积可逐步提高使其浓度呈现梯度变化,最后用水补足至20 μL。
电泳分离:上样之前需向上述体系中加入5 μL的50%甘油,保证样品沉于上样孔中;冰浴中电泳分离。电压,160 V;电泳时间,1 h。
成像:用FUJIFILM磷屏成像仪在荧光条件下进行成像。
1.8 细菌总RNA的抽提 收集10 mL相应时期的菌液(用2 mL离心管分两管收集),13400×g离心1 min,将上清尽量去除干净。立即用液氮速冻,放-80 ℃冻存;取出-80 ℃冻存样品,立即加入200 μL DEPC-treated ddH2O悬浮菌体,再加入50 μL 20 mg/mL的溶菌酶,室温静置10 min;加入1 mL Trizol试剂,振荡混匀器上振荡2 min,室温静置5 min;加入200 μL氯仿,振荡混匀,室温静置5 min,4 ℃、13400×g离心15 min;将750 μL水相上清小心转移到RNase-free的离心管中,加入750 μL异丙醇,室温静置10 min;4 ℃、13400×g离心10 min,小心弃上清,加入1 mL 75%的酒精,轻轻颠倒混匀;4 ℃、13400×g离心5 min,小心尽量吸去上清,在超净台内吹风烘干10 min;最后用20 μL DEPC双蒸馏水溶解RNA沉淀,放于-80 ℃备用或立即用于逆转录[17]。
1.9 实时荧光定量PCR 总RNA中DNA的去除和cDNA第一链的合成参照PrimeScriptTM RT reagent Kit with gDNA Eraser试剂盒的说明书进行操作。利用UltraSYBR Mixture (with ROX)试剂进行荧光定量PCR,以16S rRNA作为内参基因。引物对QyvcPQ F/QyvcPQ R及Q16S rRNA F/Q16S rRNA R见表 2。根据得到的原始Ct值,利用ΔΔCt法计算转录量[18]。
1.10 归巢内切酶Ⅰ-SceⅠ介导的基因敲除方法 本实验室利用归巢内切酶Ⅰ-SceⅠ介导的基因敲除方法,建立了苏云金芽胞杆菌中的无痕敲除方法。具体原理及操作步骤参照本实验室已发表的相关论文[8, 14, 19-21]。
2 结果和分析 2.1 YvcP正调控PkapD的活性 本实验室已经采用细菌单杂交和实时荧光定量PCR两种方法证实了YvcP能够调控kapD基因的转录[8]。为了进一步验证YvcP对kapD的调控,我们又利用β-半乳糖苷酶实验检测BMB171和ΔyvcPQ菌株在GYS培养基中培养17 h时PkapD的活性差异。
首先,以BMB171的基因组DNA为模板,PkapD F/PkapD R为引物(表 2),PCR扩增kapD的启动子区DNA片段,用PkapD表示。扩增的片段经测序验证正确后,用NcoⅠ和BamHⅠ双酶切,然后将回收得到的片段插入到pHT1K-lacZ载体上,得到载体pHT1K-PkapD-lacZ。将pHT1K-PkapD-lacZ分别转入BMB171和ΔyvcPQ中获得菌株BMB171-pHT1K-PkapD-lacZ及ΔyvcPQ-pHT1K-PkapD-lacZ,用来测定PkapD的活性。同时将不含有启动子序列的pHT1K-lacZ转入BMB171和ΔyvcPQ中获得菌株BMB171-pHT1K-lacZ及ΔyvcPQ-pHT1K-lacZ作为负对照。图 1中将菌株与质粒分开表示,可以看出在BMB171-pHT1K-PkapD-lacZ中PkapD的活性是ΔyvcPQ-pHT1K-PkapD-lacZ中的3810倍,这表明细菌缺失yvcPQ后kapD的启动子活性显著下降,因此,进一步确证了YvcP正调控PkapD的活性。
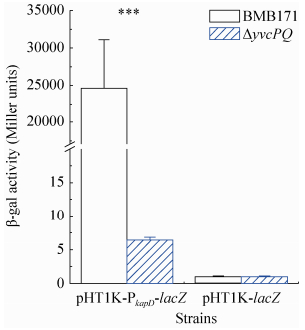 |
| 图 1. PkapD在BMB171和ΔyvcPQ中的活性差异 Figure 1. Difference of PkapD acitivities between BMB171 and ΔyvcPQ. Error bars show the variant range of the data derived from three independent biological replicates. The statistically significant differences were tested via a two-tailed student's t test. (*P < 0.05; **P < 0.01; ***P < 0.001) |
| 图选项 |
2.2 YvcPQ和KapD抑制芽胞的形成 在相差显微镜下,我们发现YvcPQ和KapD能够延迟芽胞的形成[8]。故我们通过芽胞计数实验进一步确定YvcPQ和KapD在芽胞形成过程中的作用。结果发现,在芽胞还没有完全成熟的第17 h,ΔyvcPQ和ΔkapD的芽胞计数明显高于BMB171,其中ΔyvcPQ的芽胞计数是BMB171的14倍,ΔkapD的芽胞计数是BMB171的8倍;在芽胞接近成熟的第20 h和已经成熟的第24 h,BMB171、ΔyvcPQ和ΔkapD的芽胞计数结果已经无明显的差异(图 2)。这表明在芽胞形成初期YvcP能够激活kapD基因的表达,从而抑制芽胞形成。
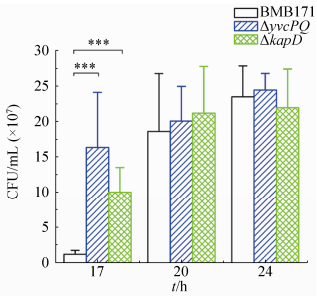 |
| 图 2. BMB171、ΔyvcPQ和ΔkapD菌株的芽胞计数 Figure 2. Spore counts in BMB171, ΔyvcPQ and ΔkapD strains. Error bars show the variant range of the data derived from three independent biological replicates. The statistically significant differences were tested via a two-tailed Student's t test. (*P < 0.05;**P < 0.01; ***P < 0.001) |
| 图选项 |
2.3 YvcP不能自调控yvcPQ操纵子的转录 一些TCS的调控机制中都包含对自身基因的调控,如响应万古霉素的LiaRS[20, 22]。因此本研究采用细菌单杂交的方法来检测YvcP是否能够自调控。细菌单杂交技术的3个基本元件是目的蛋白表达载体pTRG、报告载体pBXcmT和宿主筛选菌株E. coli XR。如果目的蛋白(DBD)与DNA (DBD binding site)能够结合,则相应的共转化菌株就能够在含有3AT (3-氨基-1, 2, 4-三氮唑)和Str的M9平板上生长。
图 3显示,所有共转化菌株都能够在不含有3AT和Str的M9培养基固体平板上生长,只有正对照可以在含有3AT和Str的M9培养基固体平板上生长,而pTRG-yvcP/pBX-PyvcPQ,自激活菌株(见表 1)和负对照菌株均不能在含有3AT和Str的M9培养基固体平板上生长,这说明YvcP与PyvcPQ不能结合,因此YvcP不能够调控自身基因的启动子。
 |
| 图 3. 细菌单杂交实验验证YvcP与PyvcPQ的结合 Figure 3. Bacterial one-hybrid assays to investigate the interaction between YvcP and PyvcPQ. +: positive control, Rv2031p/Rv3133c-hybrid strain; -: negative control, pTRG/pBX strain. |
| 图选项 |
2.4 yvcPQ操纵子的转录受调控因子AbrB的激活 细菌单杂交实验验证了YvcP不能够自调控,那么是什么转录调控元件来调节yvcPQ的转录?在B. subtilis中,形成芽胞是由于Spo0A被磷酸化后激活了芽胞形成相关sigma因子的转录,此外,磷酸化的Spo0A也能够抑制负调控芽胞形成的转录调控因子AbrB的转录。所以本研究将AbrB列为调控yvcPQ的候选蛋白,采用体外的EMSA实验和体内的实时荧光定量PCR实验来检测AbrB能否调控yvcPQ。
BL21-pET-abrB经培养,IPTG诱导,超声破碎后用Ni-NTA柱纯化,获得AbrB蛋白,与FAM标记的yvcPQ的启动子区DNA PyvcPQ混合孵育,然后用PAGE检测AbrB与PyvcPQ的结合情况,结果显示,AbrB蛋白能使PyvcPQ发生明显的阻滞迁移,且随着蛋白浓度的增加,阻滞迁移效果更加明显(图 4)。说明AbrB在体外可以与yvcPQ启动子结合。
 |
| 图 4. EMSA实验证实AbrB可以与PyvcPQ结合 Figure 4. Verification that AbrB binds to PyvcPQ by EMSA. 0: without AbrB, negative control; 1: 1 μL AbrB; 2: 2 μL AbrB; 3: 4 μL AbrB; 4: 8 μL AbrB. |
| 图选项 |
随后本研究采用实时荧光定量PCR的方法在体内检测了AbrB对yvcPQ的转录调控。首先利用归巢内切酶Ⅰ-Sce Ⅰ介导的基因敲除方法构建了abrB缺失突变株ΔabrB。将BMB171和ΔabrB在GYS培养基中培养至对数期,然后用TRIzol分别提取总RNA,如图 5-A所示,提取的RNA质量很好,可以用于实时荧光定量PCR。实时荧光定量PCR结果显示,BMB171中的yvcPQ的转录量是ΔabrB中的1.7倍(图 5-B),说明AbrB在体内能够激活yvcPQ的转录。
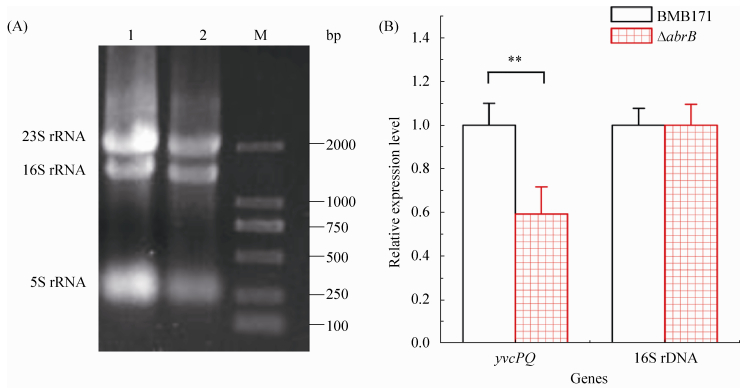 |
| 图 5. ΔabrB和BMB171中yvcPQ操纵子转录量的比较 Figure 5. Transcription analysis of yvcPQ in ΔabrB and BMB171. A: electrophoretogram of the total RNAs. 1: total RNA extracted from BMB171; 2: total RNA extracted from ΔyvcPQ; M: DL2000 DNA ladder marker. B: comparision of yvcPQ transcription level in ΔabrB with that in BMB171. Error bars show the variant range of the data derived from three independent biological replicates. The statistically significant differences were tested via a two-tailed Student's t test. (*P < 0.05; **P < 0.01; ***P < 0.001) |
| 图选项 |
3 讨论和结论 B. subtilis形成芽胞主要是由于HK KinA和KinB能够感应某种信号刺激(比如夸霉素和营徳养匮乏等)并发生自磷酸化,然后将磷酸基团经过RR Spo0F和Spo0B,最终传递给Spo0A[23],Spo0A被磷酸化后抑制调控因子AbrB的转录,解除AbrB对芽胞形成的关键因子σH基因的抑制,使σH正调控其他与芽胞形成相关的σ因子的基因的表达,最终形成芽胞[24-25],而KapD能够抑制芽胞形成的KinA途径[7]。因此,参考B. subtilis中芽胞形成机制,我们分析了B. thuringiensis中抑制芽胞形成的可能的分子机制。
当细胞外存在某种抑制芽胞形成的信号刺激时,位于细胞膜的YvcQ能够感应这种刺激,然后通过磷酸传递激活细胞质中的RR YvcP,使YvcP正调控kapD的转录,从而使KapD抑制KinA到Spo0A的磷酸传递,没有磷酸化的Spo0A不再抑制abrB基因的转录,导致AbrB通过抑制σH基因的转录来抑制芽胞形成相关的sigma因子的基因的表达,最终抑制芽胞的形成,此时,AbrB还能够正调控yvcPQ的转录,提高细胞内的YvcP的水平,增强YvcP对kapD的转录激活,进而增强对芽胞形成的抑制作用(图 6-A);当细胞外的信号刺激减弱或消失时,YvcPQ不能够被激活,导致kapD的转录量下降,此时磷酸基团能够从KinA传递到Spo0A,使Spo0A被磷酸化激活,此时Spo0A通过抑制abrB基因的转录,解除AbrB对σH基因的转录抑制,使σH正调控芽胞形成相关的σ因子的基因的表达,最终形成芽胞,同时磷酸化的Spo0A也能够通过抑制abrB基因的转录,来抑制yvcPQ的转录,使细胞内KapD水平下降,减弱其对芽胞形成的抑制作用,保证芽胞顺利的形成(图 6-B)。
 |
| 图 6. AbrB-YvcPQ-KapD对芽胞形成的抑制 Figure 6. Inhibition of sporulation by AbrB-YvcPQ-KapD. |
| 图选项 |
参考文献
| [1] | West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems.Trends in Biochemical Sciences, 2001, 26(6): 369–376DOI:10.1016/S0968-0004(01)01852-7. |
| [2] | Bourret RB, Silversmith RE. Two-component signal transduction.Current Opinion in Microbiology, 2010, 13(2): 113–115DOI:10.1016/j.mib.2010.02.003. |
| [3] | Szurmant H, Hoch JA. Interaction fidelity in two-component signaling.Current Opinion in Microbiology, 2010, 13(2): 190–197DOI:10.1016/j.mib.2010.01.007. |
| [4] | Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis:the regulatory network of the bacitracin stimulon.Molecular Microbiology, 2003, 50(5): 1591–1604DOI:10.1046/j.1365-2958.2003.03786.x. |
| [5] | Procaccini A, Lunt B, Szurmant H, Hwa T, Weigt M. Dissecting the specificity of protein-protein interaction in bacterial two-component signaling:orphans and crosstalks.PLoS One, 2011, 6(5): e19729DOI:10.1371/journal.pone.0019729. |
| [6] | Rietk?tter E, Hoyer D, Mascher T. Bacitracin sensing in Bacillus subtilis.Molecular Microbiology, 2008, 68(3): 768–785DOI:10.1111/j.1365-2958.2008.06194.x. |
| [7] | Staroń A, Finkeisen DE, Mascher T. Peptide antibiotic sensing and detoxification modules of Bacillus subtilis.Antimicrobial Agents and Chemotherapy, 2011, 55(2): 515–525DOI:10.1128/AAC.00352-10. |
| [8] | Zhang SM, Li XF, Wang X, Li Z, He J. The two-component signal transduction system YvcPQ regulates the bacterial resistance to bacitracin in Bacillus thuringiensis.Archives of Microbiology, 2016, 198(8): 773–784DOI:10.1007/s00203-016-1239-z. |
| [9] | Miller DA, Suen G, Clements KD, Angert ER. The genomic basis for the evolution of a novel form of cellular reproduction in the bacterium Epulopiscium.BMC Genomics, 2012, 13(1): 265DOI:10.1186/1471-2164-13-265. |
| [10] | Guo MM, Feng H, Zhang J, Wang WQ, Wang Y, Li YQ, Gao CH, Chen HC, Feng Y, He ZG. Dissecting transcription regulatory pathways through a new bacterial one-hybrid reporter system.Genome Research, 2009, 19(7): 1301–1308DOI:10.1101/gr.086595.108. |
| [11] | Li L, Yang C, Liu ZD, Li FD, Yu ZN. Screening of acrystalliferous mutants from Bacillus thuringiensis and their transformation properties.Acta Microbiologica Sinica, 2000, 40(1): 85–90(in Chinese).李林, 杨超, 刘子铎, 李阜棣, 喻子牛. 苏云金芽孢杆菌无晶体突变株的逐级升温筛选及其转化性能.微生物学报, 2000, 40(1): 85–90. |
| [12] | Janes BK, Stibitz S. Routine markerless gene replacement in Bacillus anthracis.Infection and Immunity, 2006, 74(3): 1949–1953DOI:10.1128/IAI.74.3.1949-1953.2006. |
| [13] | Wang JP, Ai XL, Mei H, Fu Y, Chen B, Yu ZN, He J. High-throughput identification of promoters and screening of highly active promoter-5'-UTR DNA region with different characteristics from Bacillus thuringiensis.PLoS One, 2013, 8(5): e62960DOI:10.1371/journal.pone.0062960. |
| [14] | Wang X, Li Z, Li X, Qian HL, Cai X, Li XF, He J. Poly-β-hydroxybutyrate metabolism is unrelated to the sporulation and parasporal crystal protein formation in Bacillus thuringiensis.Frontiers in Microbiology, 2016, 7: 836. |
| [15] | Meng XD, Brodsky MH, Wolfe SA. A bacterial one-hybrid system for determining the DNA-binding specificity of transcription factors.Nature Biotechnology, 2005, 23(8): 988–994DOI:10.1038/nbt1120. |
| [16] | Tang Q, Li XF, Zou TT, Zhang HM, Wang YY, Gao RS, Li ZC, He J, Feng YJ. Mycobacterium smegmatis BioQ defines a new regulatory network for biotin metabolism.Molecular Microbiology, 2014, 94(5): 1006–1023DOI:10.1111/mmi.2014.94.issue-5. |
| [17] | Wang JP, Mei H, Zheng C, Qian HL, Cui C, Fu Y, Su JM, Liu ZD, Yu ZN, He J. The metabolic regulation of sporulation and parasporal crystal formation in Bacillus thuringiensis revealed by transcriptomics and proteomics.Molecular & Cellular Proteomics, 2013, 12(5): 1363–1376. |
| [18] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method.Methods, 2001, 25(4): 402–408DOI:10.1006/meth.2001.1262. |
| [19] | Zheng C, Ma Y, Wang X, Xie YQ, Ali MK, He J. Functional analysis of the sporulation-specific diadenylate cyclase CdaS in Bacillus thuringiensis.Frontiers in Microbiology, 2015, 6: 908. |
| [20] | Zhang SM, Hu YM, Fan QY, Wang X, He J. Two-component system YvqEC-dependent bacterial resistance against vancomycin in Bacillus thuringiensis.Antonie van Leeuwenhoek, 2015, 108(2): 365–376DOI:10.1007/s10482-015-0489-0. |
| [21] | Tang Q, Yin K, Qian HL, Zhao YW, Wang W, Chou SH, Fu Y, He J. Cyclic di-GMP contributes to adaption and virulence of Bacillus thuringiensis through a riboswitch-regulated collagen adhesion protein.Scientific Reports, 2016, 6: 28807DOI:10.1038/srep28807. |
| [22] | Mascher T, Zimmer SL, Smith TA, Helmann JD. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis.Antimicrobial Agents and Chemotherapy, 2004, 48(8): 2888–2896DOI:10.1128/AAC.48.8.2888-2896.2004. |
| [23] | Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis.Current Opinion in Microbiology, 2004, 7(6): 579–586DOI:10.1016/j.mib.2004.10.001. |
| [24] | Fujita M, Losick R. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A.Genes & Development, 2005, 19(18): 2236–2244. |
| [25] | Tojo S, Hirooka K, Fujita Y. Expression of KinA and KinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control.Journal of Bacteriology, 2013, 195(8): 1656–1665DOI:10.1128/JB.02131-12. |
