王世民1

 , 张艳楠2, 胡月1, 苏艳1, 冉多良1, 包晓玮2
, 张艳楠2, 胡月1, 苏艳1, 冉多良1, 包晓玮2 1.新疆农业大学动物医学学院, 新疆 乌鲁木齐 830052;
2.新疆农业大学食品科学与药学学院, 新疆 乌鲁木齐 830052
收稿日期:2015-10-21;修回日期:2016-01-05;网络出版日期:2016-06-25
资助课题:新疆维吾尔自治区高等学校科研计划项目(XJEDU2014S024)
通信作者:Tel:+86-991-8762704;E-mail:hbbfww2005@163.com
摘要: [目的] 探究荧光蛋白标签对马疱疹病毒I型(Equine herpes virus type 1, EHV-1) gD囊膜蛋白亚细胞定位的影响。[方法] 以EHV-1基因组为模板利用PCR扩增gD全基因,分别克隆至pAcGFP1-C1和pDsRed2-N1质粒,构建pAc-GFP-gD (GFP-gD)和pDs-gD-Red (gD-Red)重组质粒;将GFP基因插入gD基因信号肽序列之后并克隆至PVAX-1质粒,构建PVAX-S-GFP-gD' (S-GFP-gD')重组质粒;将Flag标签序列与gD囊膜蛋白N端序列融合后并克隆至pVAX-1表达载体,构建pVAX-Flag-gD (Flag-gD)重组质粒。将4种不同重组真核表达质粒分别转染BHK-21细胞,通过激光共聚焦显微镜对不同融合蛋白gD进行亚细胞定位。[结果] 成功构建4种不同的融合蛋白gD真核表达载体;在BHK-21细胞单独表达时,不同融合蛋白gD绝大部分都定位于高尔基体,极少量定位于细胞核内。[结论] 不同插入位点的荧光蛋白标签对gD囊膜蛋白亚细胞定位无明显影响,这对今后研究其它蛋白亚细胞定位提供参考。
关键词: EHV-1 gD囊膜蛋白 亚细胞定位 荧光蛋白标签
Impact of fluorescent protein tag on gD envelope protein subcellular localization in BHK-21 cells
Shimin Wang1

 , Yannan Zhang2, Yue Hu1, Yan Su1, Duoliang Ran1, Xiaowei Bao2
, Yannan Zhang2, Yue Hu1, Yan Su1, Duoliang Ran1, Xiaowei Bao2 1.College of Veterinary Medicine, Xinjiang Agricultural University, Urumqi 830052, Xinjiang Uyghur Autonomous Region, China;
2.College of Food Science and Pharmacy, Xinjiang Agricultural University, Urumqi 830052, Xinjiang Uyghur Autonomous Region, China
Abstract: [Objective] The fluorescent protein and gD envelope protein of equine herpes virus type 1 (EHV-1) were used to study the impact of tags on gD protein subcellular localization in BHK-21 cells. [Methods] With the EHV-1 genome as a template, the gD complete gene was amplified by PCR technique. The product of PCR was cloned to pAcGFP1-C1 and pDsRed2-N1 plasmids. The recombinant plasmids were designated as pAc-GFP-gD (GFP-gD) and pDs-gD-Red (gD-Red). The GFP gene was inserted into the posterior position of gD gene signal peptide sequence. The modified gD gene signal peptide sequence was cloned to pVAX-1 plasmid, so that pVAX-S-GFP-gD' (S-GFPgD') recombinant plasmid was constructed. Meanwhile, the flag tag was added to N-terminal of gD sequence and they were cloned to pVAX-1 expression vector for constructing pVAX-Flag-gD recombinant plasmid. The BHK-21 cells were transfected with the 4 different recombinant plasmids and the subcellular localizations of fusion proteins were determined by lasar confocal scan microscopy. [Results] Four eukaryotic expression vectors were constructed successfully. In BHK-21 cells, the vast majority of gD envelope proteins was localized in Golgi, and a small amount of gD was localized in the nucleus. [Conclusion] Our finding reveals that the fluorescent protein of different insertion sites has no significant effects on the subcellular localization of gD, and provides a useful reference for other researchers.
Key words: EHV-1 gD envelope protein subcellular localization fluorescent protein tag
马疱疹病毒(equine herpesviruses,EHV)属α疱疹病毒亚科,是具有嚢膜的双股线状DNA病毒,对外界抵抗力较强,主要有5个亚型,其中EHV-1对马属动物危害较大。马疱疹病毒感染机体后经常无临床症状并呈潜伏状态,感染动物终身携带病毒,当机体抵抗力下降时反复发作[1]。一旦发病能引起马急性呼吸道感染、神经型瘫痪和孕马流产等不同症状,对养马业造成严重的经济损失,被我国农业部列为二类动物传染病。但遗憾的是,到目前为止还没有一种疫苗能够有效预防该病的发生[2]。
与其他病毒相比,疱疹病毒的二级囊膜的形成具有独特性,EHV-1同样如此。目前人们普遍认为疱疹病毒二级囊膜形成过程为“初级囊膜化—去囊膜化—再囊膜化”即:病毒核衣壳在宿主细胞核内组装完后与细胞核内膜结合形成初级囊膜(初级囊膜化),然后融合于细胞核外膜而脱去囊膜(去囊膜化),在细胞质中脱去初级囊膜的核衣壳与定位于高尔基体内的囊膜蛋白胞浆区结合,形成具有双层膜的病毒粒子(再囊膜化),该病毒粒子再与宿主细胞膜融合,经出芽或胞吐过程最终失去最外层囊膜后形成成熟的病毒粒子,有越来越多的证据支持这一模型[3-5]。
然而,疱疹病毒二级囊膜形成的具体过程还不十分清楚,有碍于该病毒新型疫苗的研究。gD囊膜蛋白作为疱疹病毒重要的囊膜蛋白之一,除了能够促进病毒入侵外,还能够与病毒内皮蛋白(tegument)作用促进病毒二级囊膜形成,这一点在HSV-1 (Herpes simplex virus type 1,HSV-1)上已得到证实[6]。因此,在生理条件下研究EHV-1 gD囊膜蛋白的亚细胞定位,为gD囊膜蛋白功能研究奠定基础,有利于研究EHV-1二级囊膜形成机制。在病毒复制过程中,多数病毒蛋白在宿主细胞内定位是动态过程,此过程受其它病毒的多种蛋白影响。为避免此影响,本研究通过在哺乳动物细胞内单独表达荧光蛋白标记的EHV-1 gD囊膜蛋白并对其进行亚细胞定位,并探讨荧光蛋白标签对gD囊膜蛋白定位的影响,为进一步研究EHV-1二级囊膜的形成过程奠定基础。
1 材料和方法 1.1 材料
1.1.1 细胞及毒株: BHK-21细胞为新疆农业大学传染病实验室储存,EHV-1毒株为新疆本地分离株。
1.1.2 主要试剂及仪器: Primer STAR HS DNA polymerase高保真酶、BamH Ⅰ、Xho Ⅰ、Pst Ⅰ内切酶、pMD-18T sample载体、T4 DNA连接酶均购自宝生物工程(大连)有限公司;pAcGFP1-C1、pDsRed2-N1表达载体购自Clontech公司;pVAX-1表达载体为本实验室保存;DH5α感受态细胞购自天根生化科技(北京)有限公司;去内毒素质粒提取试剂盒购自OMEGA公司;lipofectamineTM 2000转染试剂购自Invitrogen公司;抗Flag小鼠单抗、Golgi-Tracker Red荧光探针购自碧云天生物技术研究所;FITC标记羊抗小鼠二抗购自武汉博士德生物工程有限公司;胎牛血清、DMEM购自Gibco公司;二氧化碳培养箱购自Thermo公司;LSM510激光共聚焦显微镜购自德国Zeiss公司。
1.2 gD全基因克隆 根据GenBank发表的gD全基因序列(GenBank登录号AY464052.1)为参考序列,设计引物gD-F和gD-R (表 1),以EHV-1基因组DNA为模板进行扩增,获得大小约为1.2 kb的PCR产物。利用DNA纯化试剂盒进行纯化回收PCR产物并克隆至pMD-18T sample载体,利用CaCl2方法将连接产物转入DH5α感受态细胞并筛选阳性克隆,鉴定阳性重组质粒送往上海生物工程(上海)股份有限公司进行测序,测序无误后命名pMD-gD。
1.3 gD蛋白不同位点插入荧光蛋白重组质粒的构建 以pMD-gD质粒为模板,所需引物详见表 1,利用PCR方法扩增gD基因,克隆至不同的真核细胞定位载体中,使得gD蛋白的N端和C端分别融合GFP和RFP荧光蛋白,利用Overlap PCR方法将GFP荧光蛋白插入gD蛋白信号肽序列之后的位置(Ala36)并以Pst Ⅰ酶切位点连接gD去信号肽部分,3种重组质粒框架如图 1所示。同时,将Flag标签融合至gD蛋白N端并克隆至pVAX-1表达载体。构建的4种重组质粒分别转入DH5α感受态细胞,筛选阳性克隆,重组质粒经双酶切鉴定、测序无误后分别命名为pAc-GFP-gD、pDs-gD-Red、pVAX-S-GFP-gD’ (gD’为去除信号肽的gD蛋白)和pVAX-Flag-gD。
表 1. 本研究中所用引物 Table 1. Primers used in this study
| Plasmid | Primer Name | Sequences (5′→3′) |
| pMD-gD | gD-F | ATGGCTACCTTCAAGC |
| gD-R | TTACGGAAGCTGGGTAT | |
| pAc-GFP-gD | pAc-gDF | GGG CTCGAG CTACCTTCAAGCTTATG |
| pAc-gDR | TTT GGATCC CGGAAGCTGGGTATATT | |
| pDs-gD-Red | pDs-gDF | GGGCTCGAGGCTACCTTCAAGCTTAT |
| pDs-gDR | TTG GGATCC GGAAGCTGGGTATATTT | |
| Overlap1 | GCGTTGTGCTCTCTTG TGGAACATGCGAGA | |
| pVAX-S-GFP | AAGCCAAGCGTGCGGTGAGCAAGGGCGAG | |
| Overlap2 | GGATCC ATTATGGCTACCTTCAAGCTTATGA | |
| TGGATGGACGTTTGGTTTTTGCCATGGCAATCGC | ||
| GATCTTGA GCGTTGTGCTCTCTTG | ||
| Overlap R | CTGCAG CTTGTACAGCTCGTCCATG | |
| pVAX-S-GFP-gD’ | pVAX-gDF | CTGCAG GTTCGAGGACGCCAG |
| pVAX-gDR | CTCGAG CTACGGAAGCTGGGTAT | |
| pVAX-Flag-gD | Flag-gDF | GCG GGATCC ATGGACTACAAGGACGACGATGACAAGGCTACCTTCAAGC |
| Flag-gDR | CCC CTCGAG TTACGGAAGCTGGGTAT |
表选项
 |
| 图 1. 不同位点插入荧光标签构建重组融合gD蛋白 Figure 1. Schematic diagram of different insertion sites of fluorescent protein tag in recombinant glycoprotein D. |
| 图选项 |
1.4 重组质粒转染至BHK-21细胞 利用去内毒素质粒提取试剂盒提取pAc-GFP-gD、pDs-gD-Red、pVAX-S-GFP-gD’、pVAX-Flag-gD 4种重组质粒及空载体,按照lipofectamineTM 2000转染试剂说明书,通过脂质体将重组质粒分别转染至BHK-21细胞,将pAcGFP1-C1、pDsRed2-N1空载体设为对照组,转染于无血清MEM培养基37 ℃、5% CO2继续培养12 h。
1.5 荧光探针标记Golgi体及间接免疫荧光
1.5.1 Golgi-Tracker Red荧光探针染色活细胞: 试验前在24孔细胞培养板中加入灭菌的盖玻片,转染24 h后吸取培养液后用Hanks平衡液洗细胞2次后,加入Golgi-Tracker Red荧光探针工作液4 ℃进行孵育30 min,用Hanks平衡液再次洗涤细胞后,加入含10%胎牛血清MEM培养基37 ℃、5% CO2培养30 min。对染色的pAc-GFP-gD、pVAX-S-GFP-gD’质粒转染细胞及空白细胞,利用抗荧光淬灭封片剂进行封片。
1.5.2 间接免疫荧光: 对染色的pVAX-Flag-gD质粒阳性细胞,4%多聚甲醛室温固定30 min,经0.5% Triton X-100室温透化10 min后,利用10%脱脂奶粉进行室温封闭30 min后,以鼠源抗Flag单抗(1 : 1000)为一抗室温孵育1 h,FITC标记的山羊抗鼠抗体(1 : 50)为二抗室温避光孵育30 min。处理好的细胞利用抗荧光淬灭封片剂进行封片。
1.6 gD囊膜蛋白亚细胞定位观察及分析 待置于4 ℃冰箱样品封片剂凝固后,利用激光扫描共聚焦显微镜(63×water)对样品进行扫描,结果用LSM Image Browser软件进行分析。
2 结果和分析 2.1 gD全基因克隆结果 PCR产物经1.5%琼脂糖凝胶电泳,在1.2 kb附近出现条带,与预期1209 bp相符,如图 2所示。
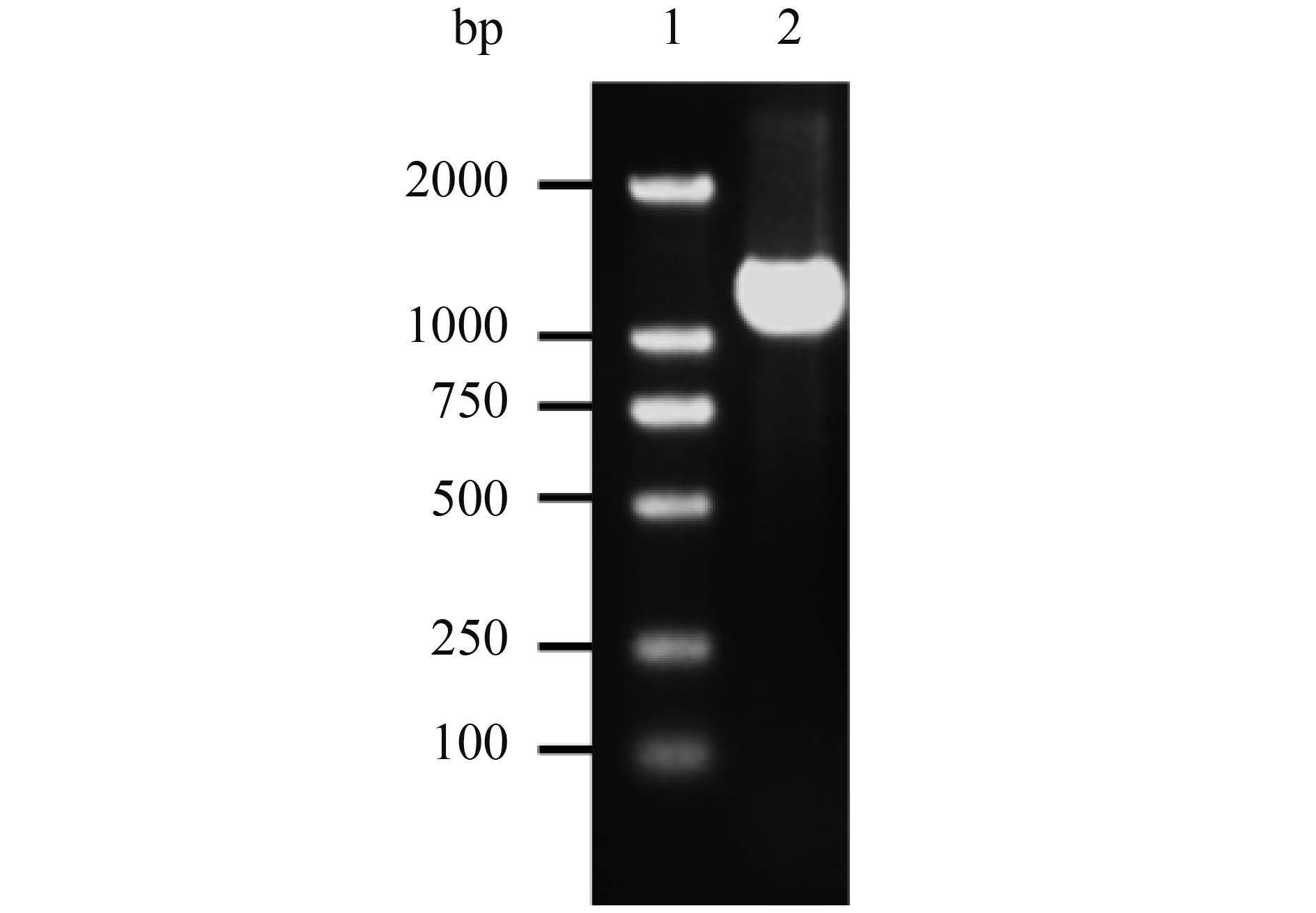 |
| 图 2. gD全基因PCR产物电泳结果 Figure 2. Electrophoresis result of gD complete gene. 1: DL2000 marker; 2: gD gene PCR product. |
| 图选项 |
2.2 4种重组质粒鉴定结果 重组质粒pAc-GFP-gD、pDs-gD-Red和pVAX-Flag-gD分别经BamH Ⅰ、Xho Ⅰ双酶切37 ℃水浴过夜;重组质粒pVAX-S-GFP-gD’经BamH Ⅰ、Pst Ⅰ、Xho Ⅰ三酶切水浴过夜。酶切产物经1.5%琼脂糖凝胶电泳,pAc-GFP-gD、pDs-gD-Red酶切产物在4.7 kb和1.2 kb附近出现条带(如图 3、4),分别与载体片段和目的基因大小相符;pVAX-S-GFP-gD’酶切产物在3.0、1.1、0.8 kb附件出现条带,分别与载体片段3.0 kb、去信号肽gD片段1103 bp及带gD信号肽的GFP片段837 bp大小相符(图 5)。
 |
| 图 3. pAc-GFP-gD,pDs-gD-Red质粒双酶切电泳结果 Figure 3. Electrophoresis results of pAc-GFP-gD and pDs-gD-Red recombinant plasmidst reated with double restriction endonuclease. 1: DL10000 marker; 2: pAcGFP1-C1 empty vector/BamH Ⅰ and Xho Ⅰ; 3: pAc-GFP-gD plasmid/BamH Ⅰ and Xho Ⅰ; 4: DL 2000 marker; 5: DL10000 maker; 6: pDsRed2-N1 empty vector/BamH Ⅰ and Xho Ⅰ; 7: pDs-gD-Red plasmid/BamH Ⅰ and Xho Ⅰ; 8: DL2000 marker. |
| 图选项 |
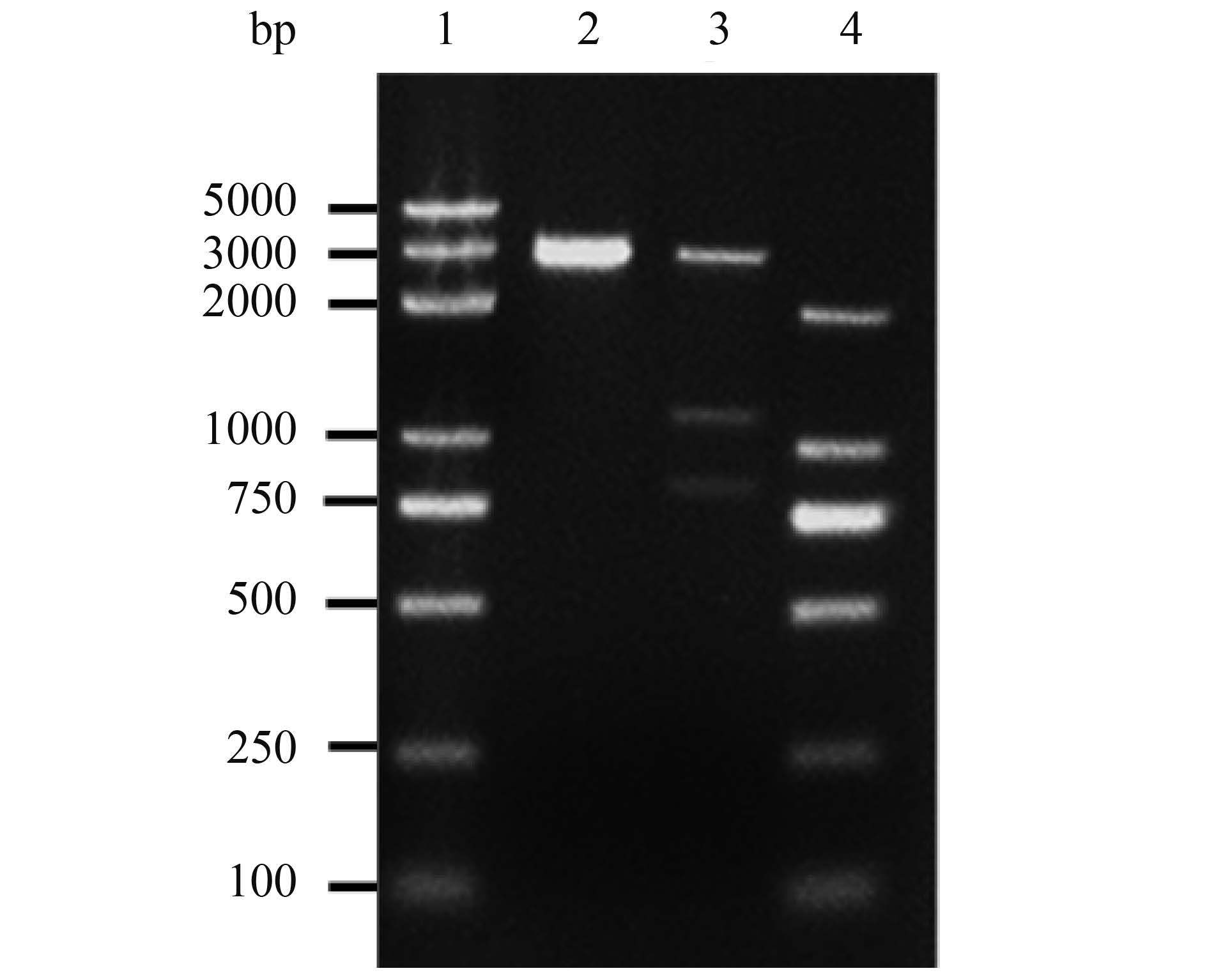 |
| 图 4. pVAX-S-GFP-gD重组质粒三酶切电泳结果 Figure 4. Electrophoresis results of pVAX-S-GFP-gD recombinant plasmid treated with triple restriction endonuclease. 1: DL5000 marker; 2: pVAX-1 empty vector/BamH Ⅰ, Pst Ⅰ and Xho Ⅰ; 3: pVAX-S-GFP-gD recombinant plasmid/BamH Ⅰ, Pst Ⅰ and Xho Ⅰ; 4: DL2000 marker. |
| 图选项 |
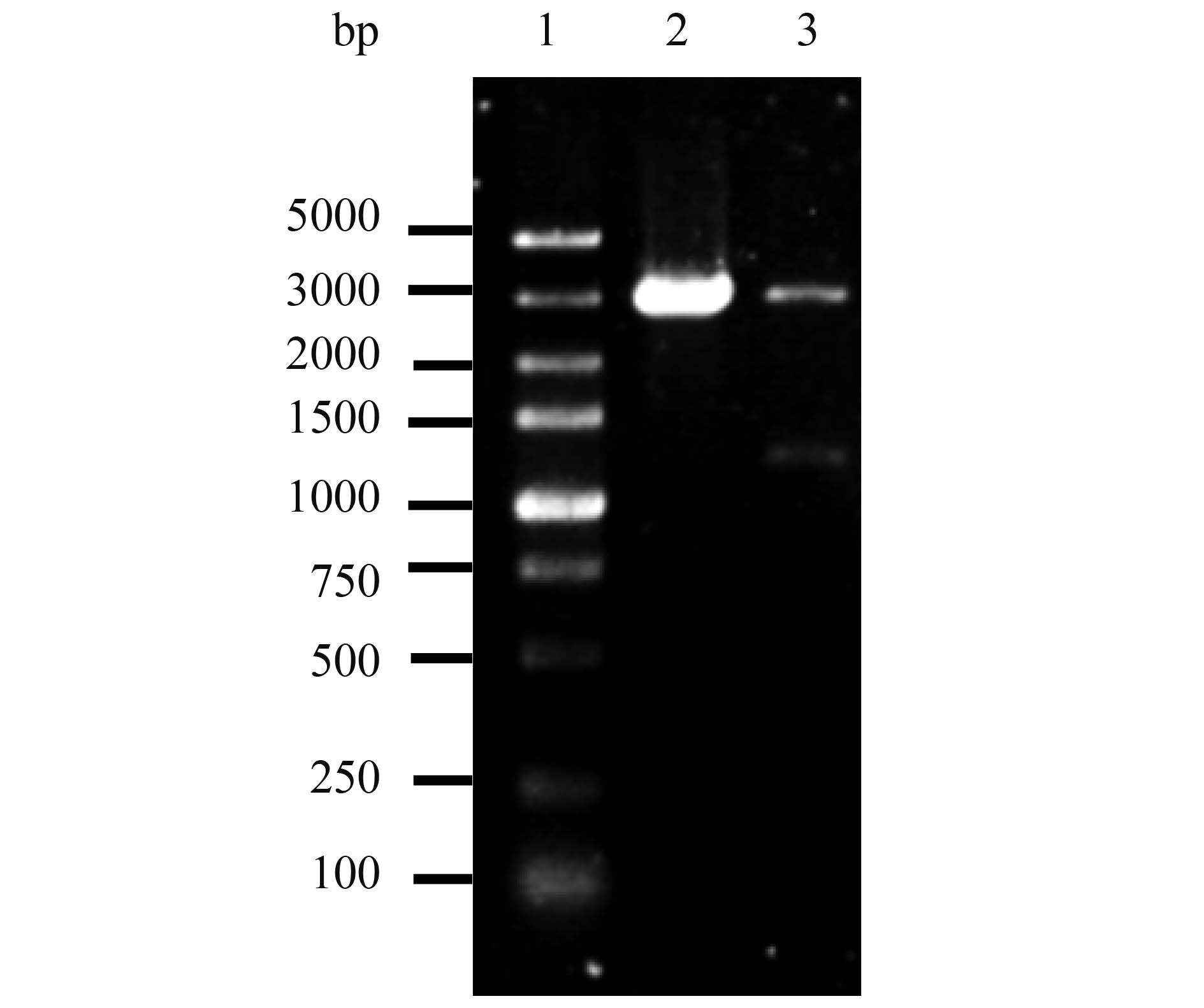 |
| 图 5. pVAX-Flag-gD重组质粒酶切电泳结果 Figure 5. Electrophoresis results of pVAX-Flag-gD recombinant plasmid treated with double restriction endonuclease. 1: DL 5000 marker; 2: pVAX-1 empty vector/BamH Ⅰ and Xho Ⅰ; 3: pVAX-Flag-gD recombinant plasmid/BamH Ⅰ and Xho Ⅰ. |
| 图选项 |
2.3 亚细胞定位结果 激光共聚焦扫描结果表明,本研究中4种不同类型的gD囊膜蛋白亚细胞定位结果无显著区别,主要定位于宿主细胞高尔基体,少量定位于宿主细胞细胞核。如图 6所示,红色荧光为高尔基体荧光探针标记细胞高尔基体或红色荧光蛋白RFP发射荧光;绿色荧光代表绿色荧光蛋白GFP发射荧光;黄色代表共定位;黑白图片为BHK-21细胞的DIC照片,其中对照组pAcGFP1-C1、pDsRed2-N1空载体所表达的GFP和RFP都分布于整个细胞内。
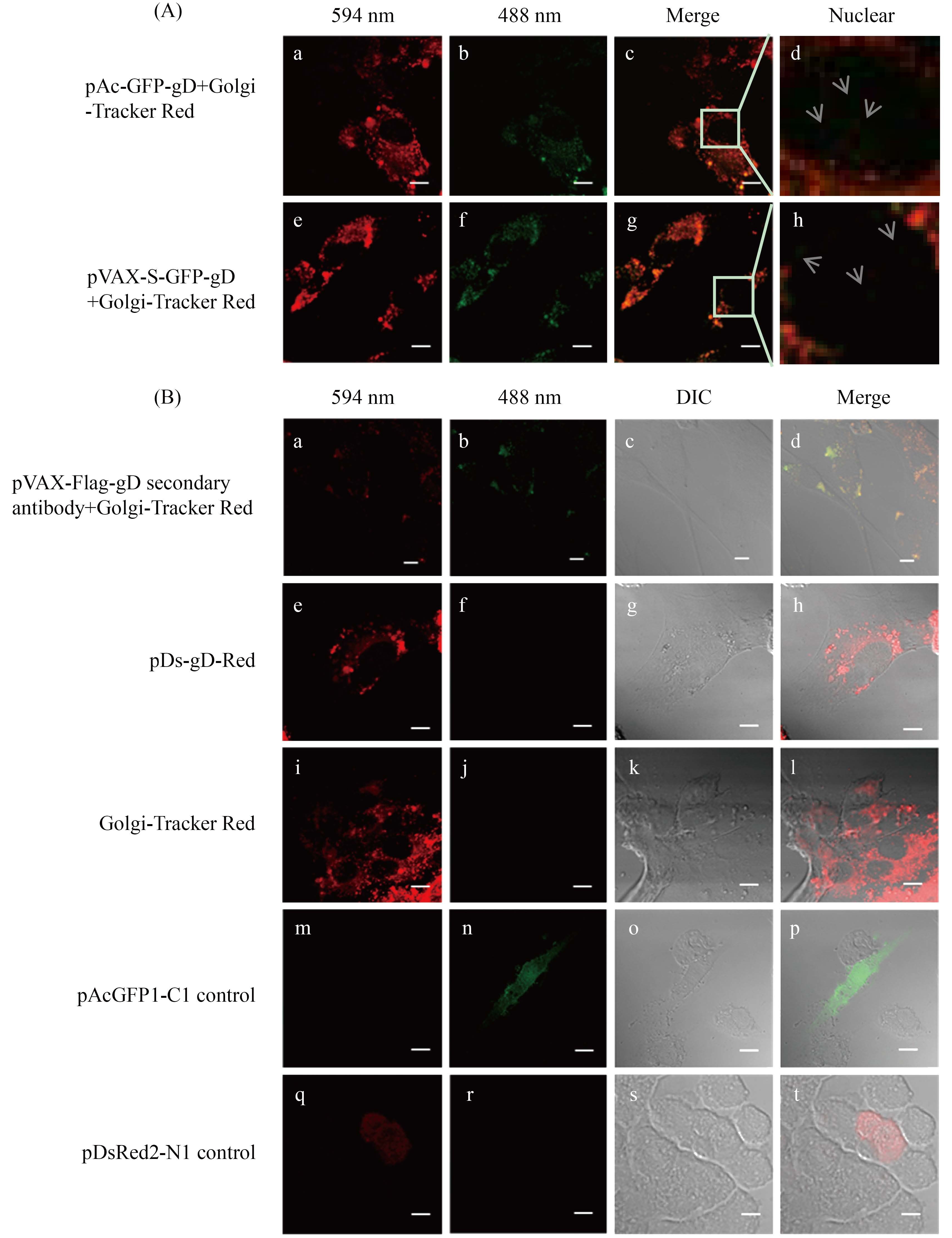 |
| 图 6. 共聚焦显微镜下观察gD囊膜蛋白在BHK-21中亚细胞定位结果(标尺长度=10 μm) Figure 6. Results of gD envelope protein subcellular localization in BHK-21 cell by cofocal (Scale bar=10 μm). A: gD envelope protein mainly localized at Golgi apparatus, and spotted in nuclear of BHK-21 cell. Localization of GFP-gD protein expressed by pAc-GFP-gD vector (a-d), S-GFP-gD protein expressed by pVAX-S-GFP-gD vector (e-h). B: analysis of gD envelope protein subcellular localization in BHK-21 cell. Localization of immunofluorescence stained Flag-gD (a-d) and gD-Red protein expressed by pDs-gD-Red vector (e-h); Golgi-Tracker Red control group (i-l), pAcGFP1-C1 (m-p) and pDsRed2-N1 (q-t) empty vector control group. |
| 图选项 |
3 讨论 疱疹病毒gD囊膜蛋白能与宿主细胞的多个细胞膜受体结合,启动膜融合过程,促进病毒入侵宿主细胞[7-13];同时gD囊膜蛋白与病毒的成熟也有关,能够与病毒自身某些内皮蛋白(tegument)相互作用,促进病毒二级囊膜的形成[6, 14-16]。在病毒复制周期中,病毒蛋白在宿主细胞内的定位不断发生变化,并最终组装成成熟的病毒颗粒后离开宿主细胞。因此,本研究为避免EHV-1的其它蛋白与gD囊膜蛋白相互作用而产生影响,单独表达荧光蛋白标记的gD囊膜蛋白,观察转染24 h后对gD囊膜蛋白亚细胞定位。
gD囊膜蛋白的N端和C端都具有信号序列,对蛋白跨膜功能上起到重要作用,Wellington等通过氨基酸测序方法证明该蛋白信号肽切割位点位于Arg35和Ala36之间[17]。因此,在本实验中将荧光蛋白GFP或RFP分别融合至gD的N端、C端和信号肽之后的不同位点,并以N端带有Flag标签的gD蛋白作为对照组,对融合的荧光蛋白标签对gD囊膜蛋白亚细胞定位是否产生影响并对其进行分析。利用激光共聚焦显微镜通过融合荧光蛋白标签或荧光二抗对gD囊膜蛋白进行定位,结果表明:EHV-1 gD囊膜蛋白主要定位于宿主细胞高尔基体,极少量定位于细胞核内,呈斑点状分布,这有异于病毒感染时gD囊膜蛋白在宿主细胞的定位[18];融合标签蛋白并未阻碍gD囊膜蛋白信号序列的正常功能,对亚细胞定位也未产生显著影响。
通过本试验结果表明,gD囊膜蛋白在没有其它病毒蛋白作用下,会滞留于宿主细胞高尔基体及小泡内,据此推断,高尔基体小泡应与病毒二级囊膜关系密切,该类型小泡很可能是病毒二级囊膜的前体。gD囊膜蛋白滞留于高尔基体内的原因,目前还不十分清楚。可能与HSV-1 gB、gE囊膜蛋白定位机制相同,通过磷酸弗林蛋白酶酸性氨基酸簇分选蛋白I (phosphofurinacidic clustersortingprotein Ⅰ,PACS-1)作用定位并滞留于高尔基体内[19]。少量的gD囊膜蛋白定位于细胞核内,可能是gD囊膜蛋白在病毒核衣壳组装或初级囊膜形成过程中同样也发挥作用。
参考文献
| [1] | Grinde B. Herpesviruses: latency and reactivation-viral strategies and host response.Journal of Oral Microbiology,2013, 5: 22766. |
| [2] | Ma GG, Azab W, Osterrieder N. Equine herpesviruses type 1 (EHV-1) and 4 (EHV-4)-masters of co-evolution and a constant threat to equids and beyond.Veterinary Microbiology,2013, 167(1/2): 123–134. |
| [3] | Meckes DG Jr, Marsh JA, Wills JW. Complex mechanisms for the packaging of the UL16 tegument protein into herpes simplex virus.Virology,2010, 398(2): 208–213. |
| [4] | Maric M, Haugo AC, Dauer W, Johnson D, Roller RJ. Nuclear envelope breakdown induced by herpes simplex virus type 1 involves the activity of viral fusion proteins.Virology,2014, 460-461: 128–137. |
| [5] | Sugimoto K, Uema M, Sagara H, Tanaka M, Sata T, Hashimoto Y, Kawaguchi Y. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1.Journal of Virology,2008, 82(11): 5198–5211. |
| [6] | Chi JHI, Harley CA, Mukhopadhyay A, Wilson DW. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids.Journal of General Virology,2005, 86(2): 253–261. |
| [7] | Sasaki M, Hasebe R, Makino Y, Suzuki T, Fukushi H, Okamoto M, Matsuda K, Taniyama H, Sawa H, Kimura T. Equine major histocompatibility complex class I molecules act as entry receptors that bind to equine herpesvirus-1 glycoprotein D.Genes to Cells,2011, 16(4): 343–357. |
| [8] | Kurtz BM, Singletary LB, Kelly SD, Frampton AR Jr. Equus caballus major histocompatibility complex class I is an entry receptor for equine herpesvirus type 1.Journal of Virology,2010, 84(18): 9027–9034. |
| [9] | Zhang N, Yan JH, Lu GW, Guo ZF, Fan Z, Wang JW, Shi Y, Qi JX, Gao GF. Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion.Nature Communications,2011, 2(12): 577. |
| [10] | Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1.PLoS Pathogens,2011, 7(9): e1002277. |
| [11] | Wang KN, Kappel JD, Canders C, Davila WF, Sayre D, Chavez M, Pesnicak L, Cohen JI. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM.Journal of Virology,2012, 86(23): 12891–12902. |
| [12] | Uchida H, Chan J, Shrivastava I, Reinhart B, Grandi P, Glorioso JC, Cohen JB. Novel mutations in gB and gH circumvent the requirement for known gD receptors in herpes simplex virus 1 entry and cell-to-cell spread.Journal of Virology,2013, 87(3): 1430–1442. |
| [13] | Clarke RW, Drews A, Browne H, Klenerman D. A single gD glycoprotein can mediate infection by Herpes simplex virus.Journal of the American Chemical Society,2013, 135(30): 11175–11180. |
| [14] | Chouljenko DV, Kim IJ, Chouljenko VN, Subramanian R, Walker JD, Kousoulas KG. Functional hierarchy of herpes simplex virus 1 viral glycoproteins in cytoplasmic virion envelopment and egress.Journal of Virology,2012, 86(8): 4262–4270. |
| [15] | Johnson DC, Wisner TW, Wright CC. Herpes simplex virus glycoproteins gB and gD function in a redundant fashion to promote secondary envelopment.Journal of Virology,2011, 85(10): 4910–4926. |
| [16] | Whittaker GR, Taylor LA, Elton DM, Giles LE, Bonass WA, Halliburton IW, Killington RA, Meredith DM. Glycoprotein 60 of equine herpesvirus type 1 is a homologue of herpes simplex virus glycoprotein D and plays a major role in penetration of cells.Journal of General Virology,1992, 73(4): 801–809. |
| [17] | Wellington JE, Gooley AA, Love DN, Whalley JM. N-terminal sequence analysis of equine herpesvirus 1 glycoproteins D and B and evidence for internal cleavage of the gene 71 product.Journal of General Virology,1996, 77(1): 75–82. |
| [18] | Flowers CC, Flowers SP, Jennings SR, O'Callaghan DJ. Synthesis and processing of equine herpesvirus 1 glycoprotein D.Virology,1995, 208(1): 9–18. |
| [19] | 刘军. PACS-1在单纯疱疹病毒I型糖蛋白B定位于分泌高尔基体网络中的作用. 第四军医大学博士学位论文, 2001. |
