包利平, 朱立, 张鑫宇, 孙怀昌


扬州大学兽医学院, 江苏省重要动物疫病与人兽共患病防控协同创新中心, 江苏 扬州 225009
收稿日期:2015-09-07;修回日期:2015-10-16;网络出版日期:2016-06-04
资助课题:江苏省高校优势学科建设项目(PAPD);江苏省前瞻性研究专项(BE2004380)
通信作者:Tel:+86-514-87979335;Fax:+86-514-87972218; E-mail:sunh@yzu.edu.cn.
摘要: [目的] 研究重组腺病毒(rAd)传送的3'非翻译区(UTR)靶向amiR3UTR对猪繁殖与呼吸综合征病毒(PRRSV)在猪肺巨噬细胞(PAM)中复制的抑制作用。[方法] 用表达amiR3UTR或对照amiRcon的腺病毒载体转染AAV-293细胞, 获得rAd-amiR3UTR-GFP 和rAd-amiRcon-GFP, 用定量RT-PCR检测amiR3UTR在rAd转导细胞中的表达, 用定量RT-PCR、Western blotting和病毒滴定检测amiR3UTR对PRRSV复制的抑制作用。[结果] 原代PAM及其细胞系3D4/163均能被rAd-amiR3UTR-GFP转导, 但前者转导效率很低;rAd-amiR3UTR-GFP转导细胞能有效表达amiR3UTR, 且表达具有剂量和时间依赖性;rAd表达的amiR3UTR能显著抑制不同毒株PRRSV在PAM细胞中的复制, 且抑制作用具有剂量依赖性。[结论] amiR3UTR能抑制不同毒株PRRSV在PAM中的复制, 其rAd有望作为抗PRRSV新策略进行深入研究。
关键词: 猪繁殖与呼吸综合征病毒 3'非翻译区 人工微小RNA 重组腺病毒 抗病毒作用
Inhibition of porcine reproductive and respiratory syndrome virus replication in porcine alveolar macrophages with the 3' UTR-targeted amiRNA
Liping Bao, Li Zhu, Xinyu Zhang, Huaichang Sun


Jiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, Jiangsu Province, China
Supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and by the SpecialProspective Research Project of Jiangsu Province, China (BE2004380)
Abstract: [Objective] To study the inhibitory effect of 3' untranslated region (UTR)-targeted artificial microRNA (amiRNA) against porcine reproductive and respiratory syndrome virus (PRRSV) replication in porcine alveolar macrophages (PAM). [Methods] Recombinant adenovirus (rAd) expressing the 3' UTR-targeted or control amiRNA and green fluorescent protein (GFP) reporter gene were generated by transfecting AAV-293 cells with the transfer vector. The expression of sequence-specific amiRNA was detected by quantitative RT-PCR. The anti-PRRSV effect of amiRNA was detected by quantitative RT-PCR, Western blotting and viral titration assay. [Results] Two rAds, namely rAd-amiR3UTR-GFP and rAd-amiRcon-GFP, were generated. Both primary PAM and 3D4/163 cells could be transduced by rAd with different transduction efficiencies. The amiR3UTR was expressed in dose-and timedependent manners in rAd-transduced PAM cells. The amiR3UTR, but not amiRcon, had significant and stable inhibitory effects against replication of three different PRRSV strains in a dose-dependent manner. [Conclusion] The rAd-delivered amiR3UTR had strong anti-PRRSV effect against different PRRSV strains and rAd-amiR3UTR-GFP could be explored further as the alternative strategy against PRRS.
Key words: porcine reproductive and respiratory syndrome virus 3' untranslated region artificial microRNA recombinant adenovirus anti-viral effect
Porcine reproductive and respiratory syndrome (PRRS) is one of the most important swine diseases characterized by reproductive failures in sows and respiratory syndromes in pigs of all age[1-2]. Although both inactivated and live-attenuated vaccines are available for PRRS control, the current vaccines failed to provide sustainable protection against heterogeneous viral strains[3]. Porcine reproductive and respiratory syndrome virus (PRRSV) is an enveloped positive-sense RNA virus within the family Arteriviridae[4]. The virus targets the cells of porcine monocyte/macrophage lineage, causing severe cell death, slow and weak antiviral responses, and/or persistent infection[5-6]. In addition, PRRSV uses several evasion strategies to escape the host immunity. As a consequence, new PRRS vaccine development faces great challenges[3].
RNA interference (RNAi) is a post-transcriptional gene silencing mechanism conserved in eukaryotes[7]. Since its discovery as a natural anti-viral mechanism, RNAi has become a feasible strategy against a variety of viral infections[8]. Furthermore, recent studies have shown that the vector-encoded artificial microRNAs (amiRNAs) are more effective than the conventional short hairpin RNA strategy[9-10]. Our previous study has shown that the plasmid vector-delivered amiRNA targeting the 5′ or 3′ untranslated region (UTR) has a potent inhibitory effect against PRRSV replication in Marc-145 cells[11]. More recently, we have shown that the exosome-delivered 3′ UTR-targeted amiRNA has strong inhibitory effect against PRRSV replication in porcine alveolar macrophages (PAM)[12]. In this study, the anti-PRRSV effect of 3′ UTR-targeted amiRNA was investigated further by transduction of PAM with recombinant adenoviral (rAd) vector.
1 Materials and Methods 1.1 Vector, cells and viruses Ad vector pShuttle-IRES-GFP[12] was constructed by cloning the internal ribosome entry sequence (IRES) of human translation initiation factor 4G and green fluorescent protein (GFP) reporter gene into pShuttle-CMV vector (Stratagene, USA). Human embryonic kidney cell line AAV-293 (Stratagene, USA) and monkey kidney cell line Marc-145 (ATCC, USA) were grown in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% non-essential amino acids (Gibco, USA). Primary PAM cells were prepared from 6-week-old SPF pigs as described previously[13]. PAM cell line 3D4/163 was generated by transfecting 3D4/21 cell line (ATCC, USA) with porcine CD163 cDNA as previously described[6]. Primary PAM and 3D4/163 cells were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% FBS, 1% non-essential amino acids and 20% L929 cells-cultured medium[13]. PRRSV strain VR-2332 (ATCC, USA) is a prototype strain of North American genotype[14]. PRRSV strain CH-1R is an attenuated vaccine strain derived from the traditional Chinese strain CH-1a[15]. Strain JX-A1 is a highly pathogenic Chinese PRRSV strain[16]. The three PRRSV strains were propagated and titrated on Marc-145 cells as previously described[17].
1.2 rAd preparation PRRSV 3′ UTR-targeted or control amiRNA expression cassette was excised from pcDNA-amiR3UTR1 or pcDNA-amiRcon[11] with restriction enzymes Kpn Ⅰ and Not Ⅰ, and ligated with pShuttle-IRES-GFP vector linearized with the same enzymes. The two rAds, namely rAd-amiR3UTR-GFP and rAd-amiRcon-GFP, were generated by transfecting AAV-293 cells with each rAd vector (Figure1-A) according to the instruction manual for AdEasyTM Adenoviral Vector System (Agilent Technologies, USA). The rAds were amplified and titrated as fluorescence forming units/mL (FFU/mL) on AAV-293 cells. The amiRNAs encoded by rAd-amiR3UTR-GFP and rAd-amiRcon-GFP were called amiR3UTR and amiRcon, respectively.
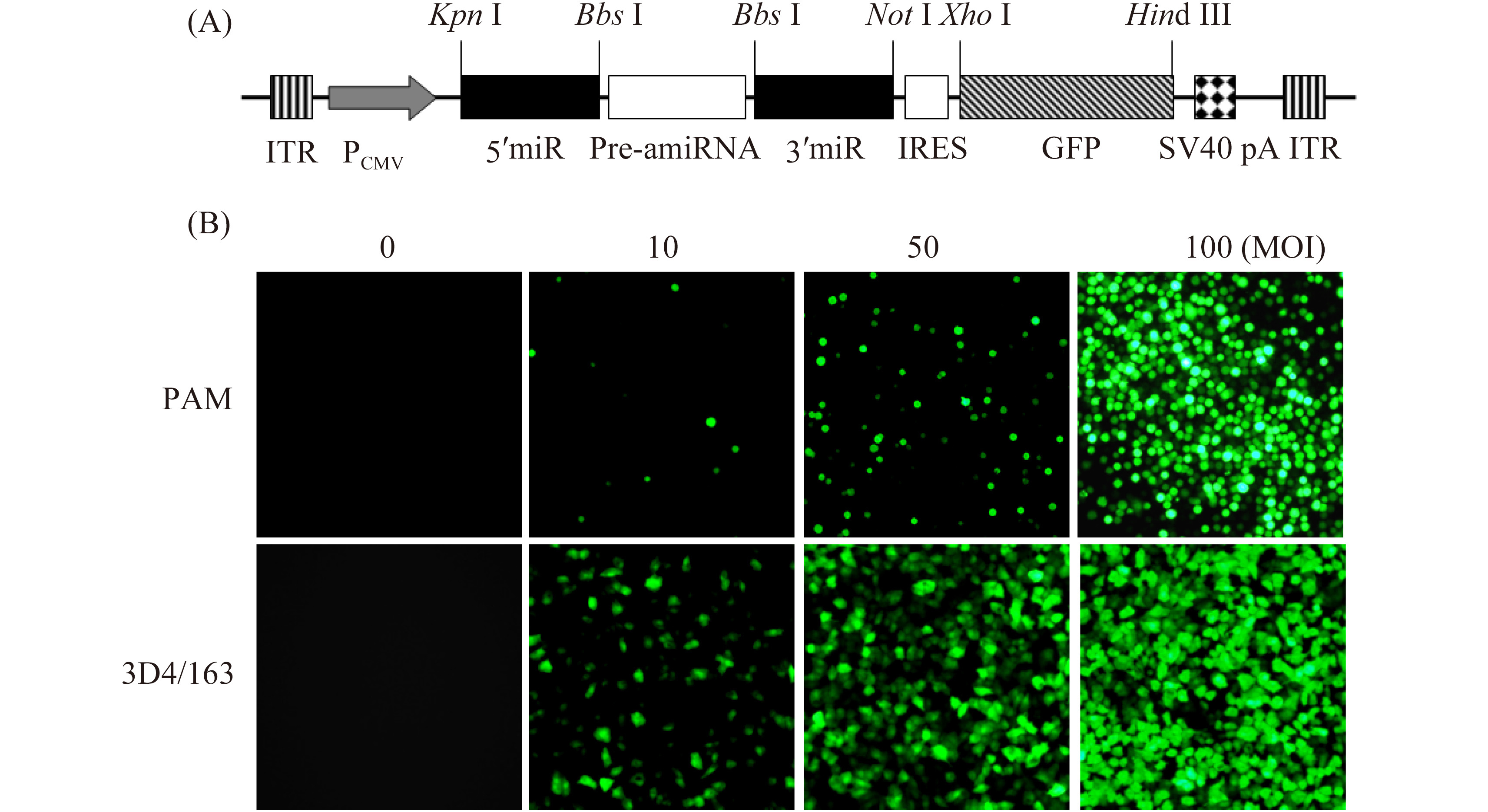 |
| 图 1. 重组腺病毒转导细胞的荧光显微镜观察 Figure 1. Fluorescent microscopy of rAd-transduced PAM. |
| 图选项 |
1.3 Cell transduction Primary PAM and 3D4/163 cells were seeded in triplicates on 24-well plates and grown to 80% confluent growth. After wash with PBS, the cells were transduced (37 ℃/1 h) with rAd-amiR3UTR-GFP or rAd-amiRcon-GFP. The cells were grown for different times in fresh medium before fluorescent microscopy, RNA extraction or PRRSV infection.
1.4 Quantitative RT-PCR for amiRNA detection Total RNA was extracted from rAd-transduced cells using RNAiso Plus (TaKaRa, China). After removal of residual cellular DNA by DNase I digestion, the amiRNA cDNA was synthesized with One Step RT-PCR Kit (Roche, China) using 100 ng of RNA and modified stem-loop RT primer (5′-GGTCGTATGCAAAGCAGGGTCCGAGGTATCCATCGCACGCATCGCACTGCATACGACCTGCCTC-3′). The SYBR Green Quantitative RT-PCR for amiR3UTR detection was performed on LightCycler? Nano System (Roche, China) using 2 μL of cDNA, the sequence-specific forward primer (5′-CCGCCGTGAATAGGTGACTTA-3′) and universal reverse primer (5′-GAGCAGGGTCCGAGGT-3′) in FastStart Essential DNA Green Master Kit (Roche, China) by following the instruction manual. The 45-cycle PCR was carried out using the following program: 95 ℃ for 10 min; 94 ℃ for 10 s, and 60 ℃ for 40 s. The standard curve was generated using quantified pMD18-T vector containing the PCR product. All PCR products were analyzed by electrophoresis on 3% agarose gels.
1.5 Quantitative RT-PCR for PRRSV ORF7 detection The real-time quantitative RT-PCR for PRRSV genomic RNA detection was performed using the primer pair of ORF7 which encodes the nucleocapsid (N) protein as previously described[12]. Briefly, primary PAM and 3D4/163 cells were transduced with different multiplicities of infection (MOI) of rAd-amiR3UTR-GFP or rAd-amiRcon-GFP. At 48 h after transduction, the cells were infected with different doses of PRRSV. At 24 h post infection, total RNA was extracted for reverse transcription and real-time quantitative PCR as described. The standard curve was generated using the plasmid containing RT-PCR product of PRRSV ORF7.
1.6 Western blotting Primary PAM and 3D4/163 cells were transduced with rAd-amiR3UTR-GFP or rAd-amiRcon-GFP, and then infected with PRRSV strain VR-2332 as described. At 24 h post infection, the cell extracts were separated on 12% SDS-PAGE gels and transferred onto PVDF membrane. The membrane was blocked overnight at 4 ℃ with 5% BSA and 0.05% Tween 20 in PBS (pH 7.4), and Western blotting was performed using pig anti-serum (1:100) recovered from natural PRRSV infection. The hybridization signal of N protein, which is immunodominant antigen and main target for serological diagnosis of PRRSV, was revealed using rabbit IgG IRDye800 conjugated antibody (1:10000; Santa Cruz Biotechnology, USA).
1.7 Statistical analysis All data were expressed as mean±SD and statistically analyzed using one way anova-test and student’s t-test.
2 Results 2.1 Transduction of PAM with rAd Primary PAM and 3D4/163 cells were transduced with different doses of rAd-amiR3UTR-GFP and grown for additional 24 h. Fluorescent microscopy showed that 3D4/163 cells were transduced more efficiently than primary PAM. At MOI 50, for example, about 70% of the rAd-transduced 3D4/163 cells were GFP-positive, compared to less than 10% of GFP-positive cells in the rAd-transduced PAM (Figure 1-B).
2.2 Dose-dependent expression of amiR3UTR in rAd-transduced PAM Primary PAM and 3D4/163 cells were transduced with different doses of rAd-amiR3UTR-GFP. At 48 h after transduction, the total RNA was extracted for amiR3UTR copy number detection by quantitative RT-PCR. An expected 90-nt amiRNA was detected in rAd-transduced cells, but not in mock-transduced cells (Figure 2). The amiRNA copy number was increased as increase of rAd MOI, reaching to the highest copy number of 104.3 or 106.8/μg RNA in primary PAM or 3D4/163 cells at MOI 100.
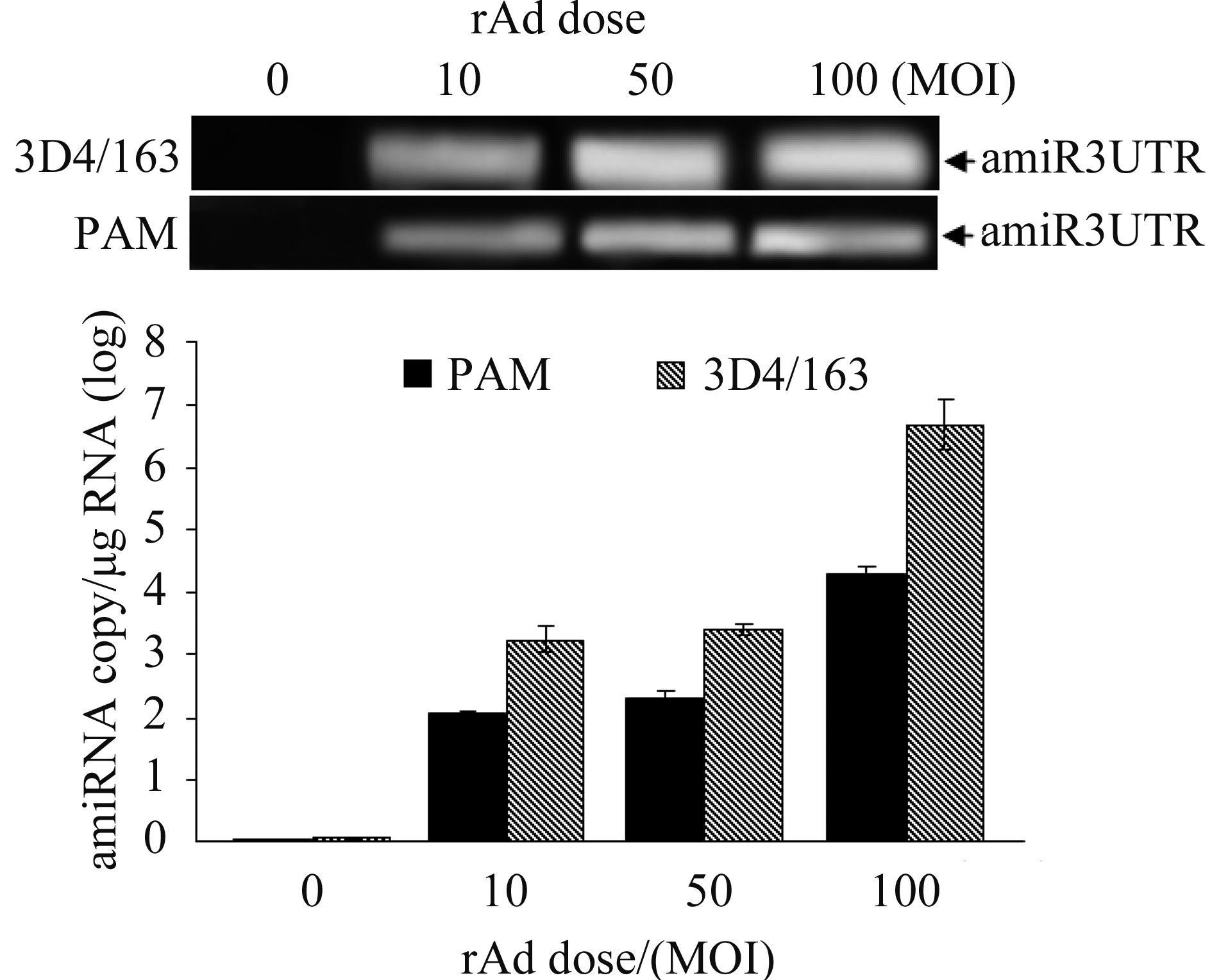 |
| 图 2. 重组腺病毒转导细胞的amiR3UTR表达检测 Figure 2. Detection of amiR3UTR expression in rAdtransduced PAM. |
| 图选项 |
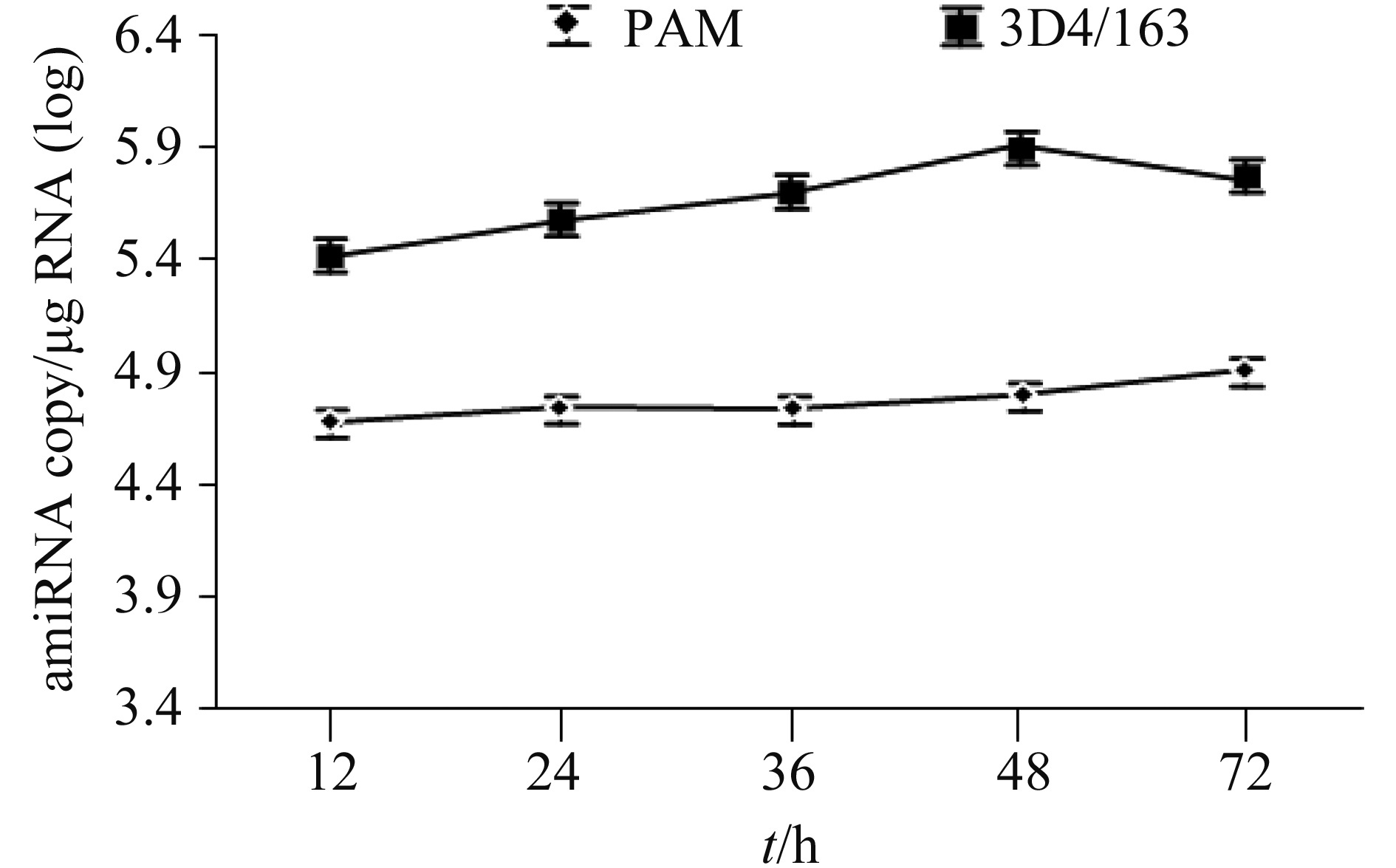
2.3 Dynamic expression of amiR3UTR in rAd-transduced PAM Primary PAM and 3D4/163 cells were transduced with rAd-amiR3UTR-GFP (MOI 100) and the total RNA was extracted at different time points after transduction. Quantitative RT-PCR showed that the amiR3UTR was expressed as early as 12 h after rAd transduction (Figure 3). The amiRNA copy number in rAd-transduced primary PAM was increased slightly as extension of incubation time, reaching to the highest copy number of 104.9/μg RNA 72 h after transduction. On the other hand, the amiRNA copy number in rAd-transduced 3D4/163 cells reached to the highest copy number of 105.9/μg RNA 48 h after transduction, which started to drop hereafter (Figure 3).
 |
| 图 3. 重组腺病毒转导细胞的amiR3UTR表达动态 Figure 3. Time course of amiR3UTR expression in rAd-transduced cells. |
| 图选项 |
2.4 Dose-dependent inhibition of PRRSV replication by amiR3UTR Two different strategies were used to evaluate the anti-PRRSV effect of amiR3UTR. First, primary PAM and 3D4/163 cells were transduced with different doses of rAd-amiR3UTR-GFP or rAd-amiRcon-GFP, and then infected with PRRSV strain VR2332 (MOI 0.5). At 24 h post infection, PRRSV ORF7 copy numbers were detected by quantitative RT-PCR. Compared to that (993, 1014 or 995/μg RNA) in the control groups, PRRSV ORF7 copy number was decreased to 925, 780 or 280/μg RNA in primary PAM (Figure 4-A) and 570, 460 or 107/μg RNA in 3D4/163 cells (Figure 4-B), respectively, following transduction with rAd-amiR3UTR-GFP at MOI 10, 50 or 100. The dose-dependent inhibitory effect of amiR3UTR against PRRSV replication was confirmed by Western blotting (Figure 4-C).
Next, the two types of cells were transduced with rAd-amiRcon-GFP or rAd-amiR3UTR-GFP (MOI 100) and then infected with PRRSV at MOI 0.1, 0.5 or 1.0. Compared to that (505, 1005 or 1230/μg RNA) in the control groups, PRRSV ORF7 copy number was decreased to 223, 459 or 615/μg RNA in rAd-amiR3UTR-GFP-transduced primary PAM (Figure 5-A) and to 81, 225 or 426/μg RNA in rAd-amiR3UTR-GFP-transduced 3D4/163 cells (Figure 5-B), respectively, following infection with PRRSV at 0.1, 0.5 or 1.0. The dose-dependent inhibitory effect of amiR3UTR against PRRSV replication was also confirmed by Western blotting (Figure 5-C).
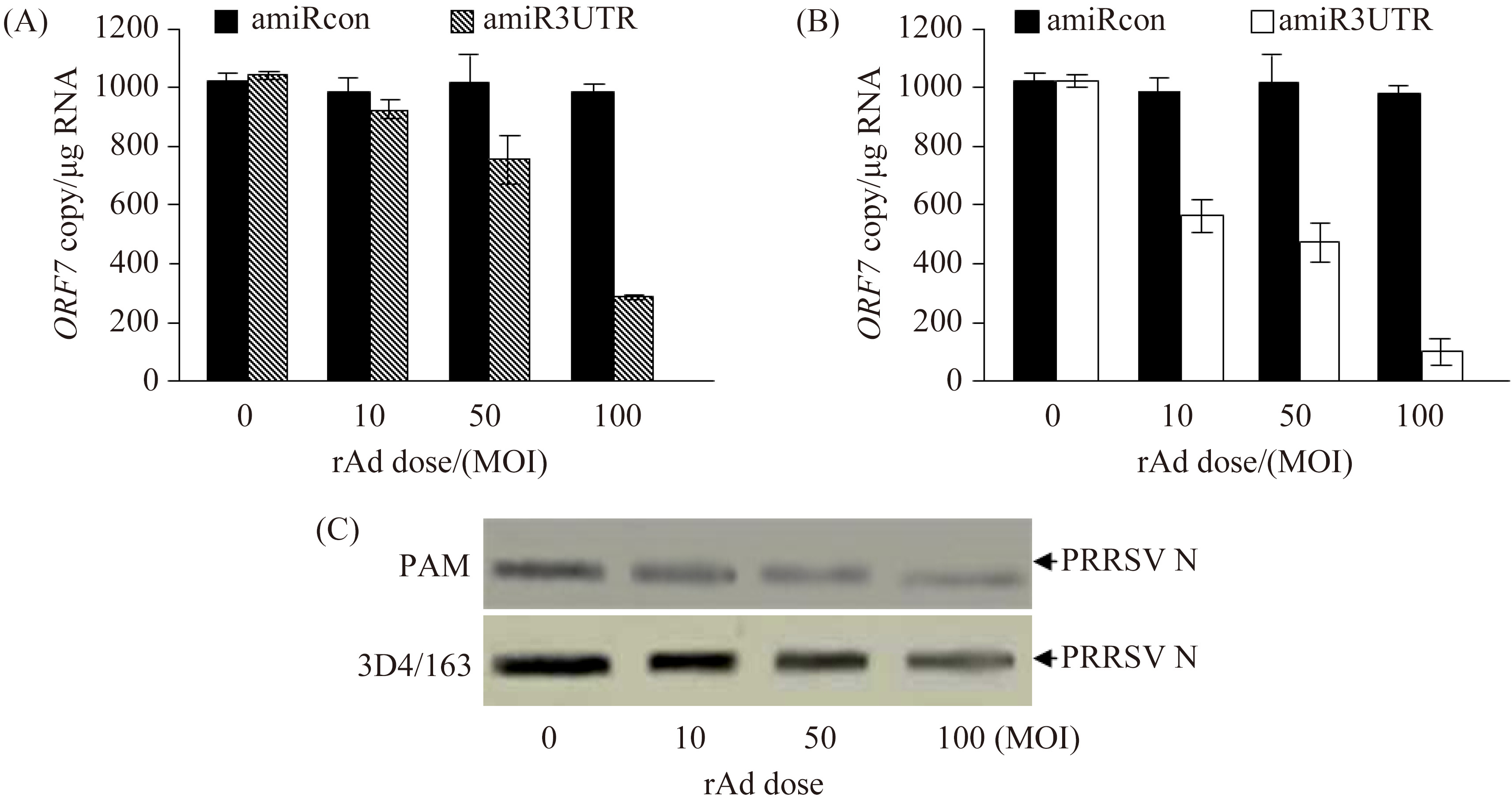 |
| 图 4. amiR3UTR抑制PRRSV复制的剂量依赖关系 Figure 4. Dose-dependent inhibition of PRRSV replication by amiR3UTR. |
| 图选项 |
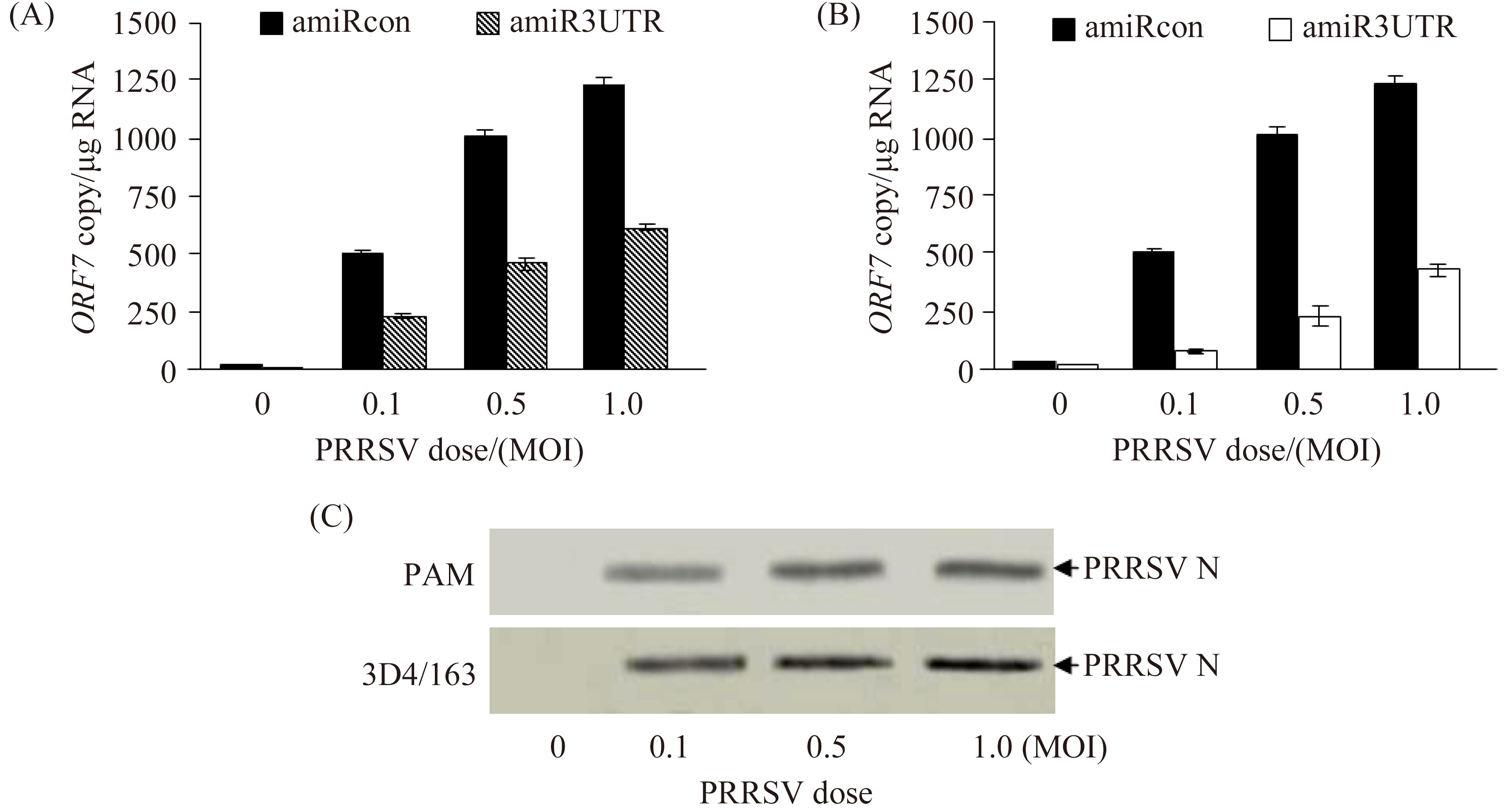 |
| 图 5. amiR3UTR抑制PRRSV复制的剂量依赖关系 Figure 5. Dose-dependent inhibition of PRRSV replication by amiR3UTR. |
| 图选项 |
2.5 Dynamic inhibition of PRRSV replication by amiR3UTR Primary PAM and 3D4/163 cells were transduced with rAd-amiR3UTR-GFP or rAd-amiRcon-GFP (MOI 100), and then infected with PRRSV strain VR 2332 (MOI 0.5). At different time points post infection, PRRSV was harvested and titrated on Marc-145 cells. As Figure 6 shows, the anti-PRRSV effect of amiR3UTR lasted for at least 96 h.
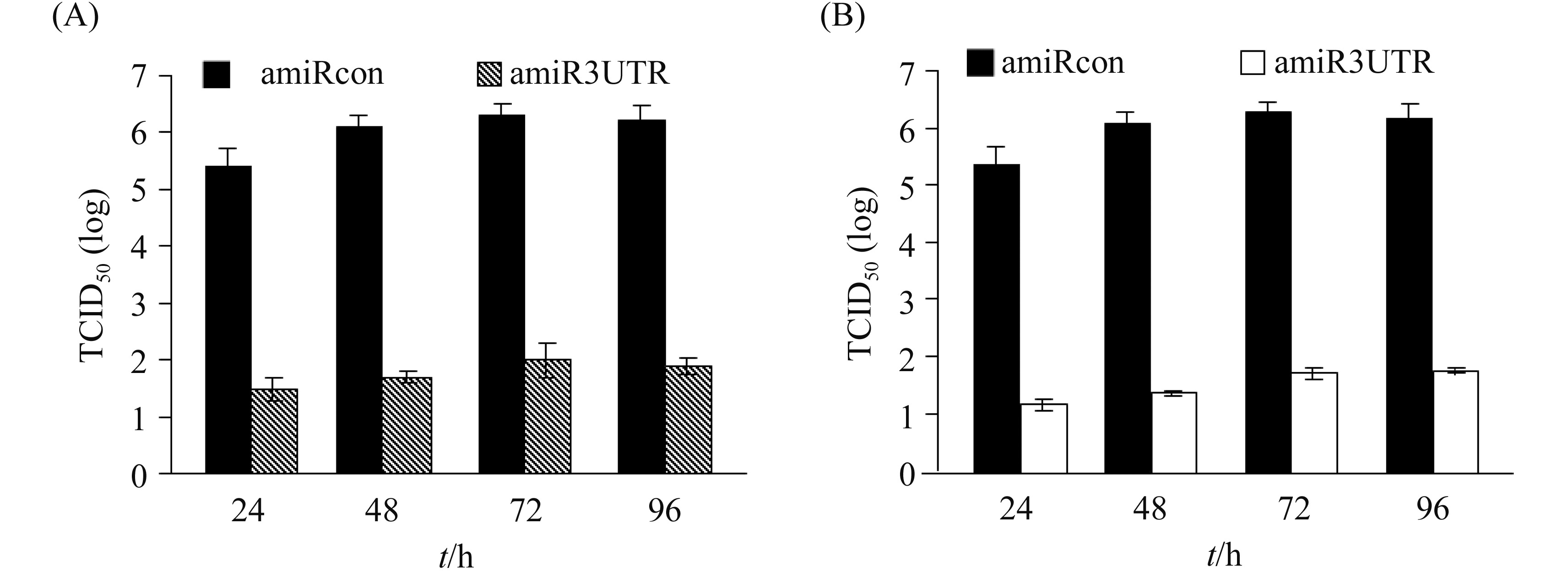 |
| 图 6. amiR3UTR抑制PRRSV复制的动态检测 Figure 6. Dynamic inhibition of PRRSV replication by amiR3UTR. |
| 图选项 |
2.6 Inhibitory effect of amiR3UTR against replication of different PRRSV strains Primary PAM and 3D4/163 cells were transduced with rAd-amiR3UTR-GFP or rAd-amiRcon-GFP, and then infected with PRRSV strain VR 2332, CH1R or JXA1 (MOI 0.5). At 24 h post infection, the three PRRSV strains were harvested and titrated on Marc-145 cells. Compared to that (5.4, 5.6 or 5.0 log TCID50) in the control groups, PRRSV titers of three strains were decreased by 3.8, 3.9 or 3.6 in rAd-amiR3UTR-GFP-transduced primary PAM (Figure 7-A) and by 3.0, 4.4 or 3.6 log TCID50 in rAd-amiR3UTR-GFP-transduced 3D4/163 cells (Figure 7-B), respectively.
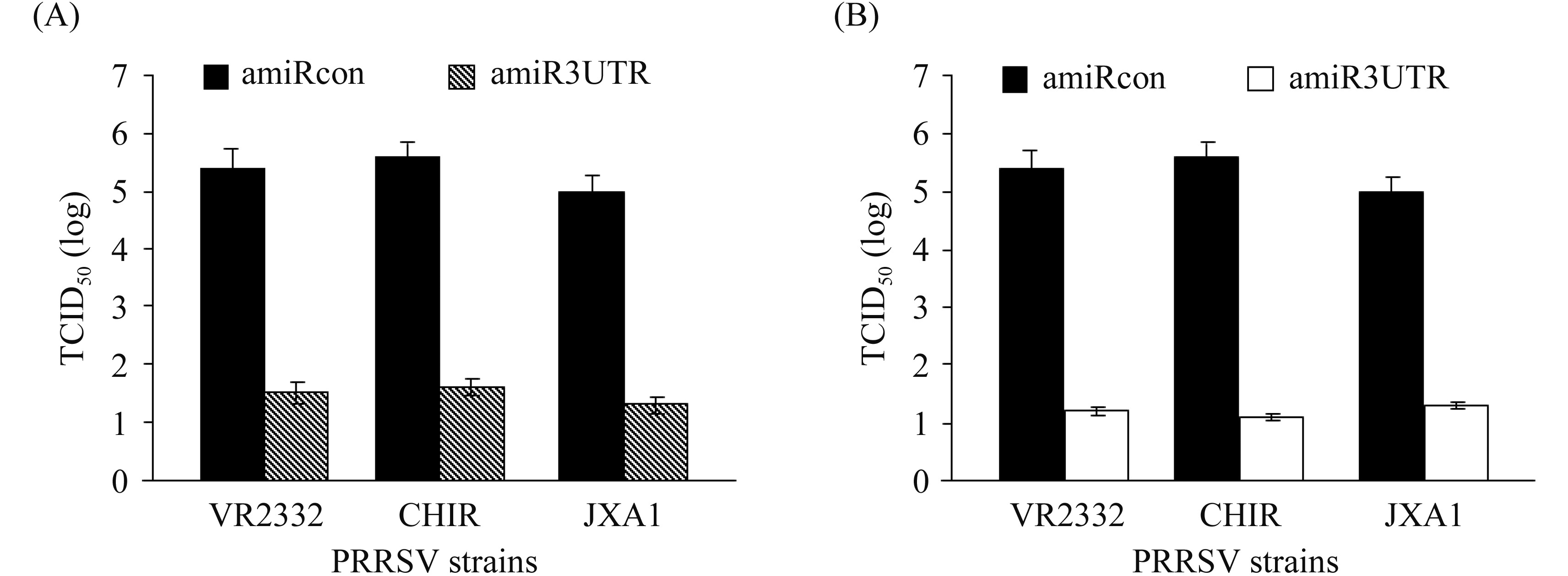 |
| 图 7. amiR3UTR对不同毒株PRRSV复制的抑制作用 Figure 7. Inhibitory effect of amiR3UTR against replication of different PRRSV strains. |
| 图选项 |
3 Discussion Our previous study has shown that plasmid vector-delivered amiRNAs targeting the 5′ or 3′ UTR have potent inhibitory effects against PRRSV replication in Marc-145 cells[11]. To make the amiRNA strategy more useful, in this study we delivered the 3′ UTR-targeted amiRNA into PAM cells with rAd-amiR3UTR-GFP, and evaluated its anti-PRRSV effect using different assays. In vitro cell transduction assay showed that primary PAM cells were highly resistant to rAd transduction with less than 5% transduction efficiency at MOI 10. One of the possible reasons could be due to the absence of high affinity Ad receptor on the primary PAM[18]. However, this could not explain much higher rAd transduction efficiency for 3D4/163 cells which are derived from primary PAM. In the light of the fact that 3D4/163 cells proliferate more quickly than primary PAM, the main reason for the resistance of primary PAM to rAd transduction could be due to their slower growth rate rather than lack of high affinity Ad receptor.
Nevertheless, our quantitative RT-PCR showed that amiR3UTR was expressed efficiently in the rAd-transduced primary PAM and 3D4/163 cells in both dose- and time-dependent manners. The different amiRNA expression levels between primary PAM and 3D4/163 cells were correlated well with their different rAd transduction efficiencies. The similar results were also obtained from PRRSV infection blocking assays. For example, both PRRSV ORF7 copy numbers and viral titers were decreased differently by transducing primary PAM or 3D4/163 cells with different doses of rAd-amiR3UTR-GFP or infection of the transduced cells with different doses of PRRSV. Nevertheless, the similar anti-PRRSV effect could be achieved by transduction of primary PAM or 3D4/163 cells with the highest dose (MOI 100) of rAd-amiR3UTR-GFP.
The time course study showed that the anti-PRRSV effect of rAd-delivered amiR3UTR lasted for at least 96 h in both primary PAM and 3D4/163 cells, which was even more stable than that in Marc-145 cells stably transfected with the amiRNA expression vector[11]. More importantly, the rAd-delivered amiR3UTR was not only effective against three different PRRSV strains, but also more stable than the plasmid-delivered amiR3UTR. Unlike the low transduction efficiency of primary PAM in vitro, however, transduction of alveolar macrophages by adenoviruses proceeds reasonably well in vivo due to the presence of surfactant proteins in the lung that can enhance viral uptake[19]. Therefore, even more potent anti-PRRSV effect of rAd-delivered amiR3UTR could be expected in vivo.
In conclusion, rAd-delivered amiR3UTR had a long lasting and stable inhibitory effect against replication of different PRRSV strains in primary PAM and cell line 3D4/163. These data warrant us to explore the in vivo usefulness of rAd vector as an alternative strategy against PRRS.
参考文献
| [1] | Lunney JK, Benfield DA, Rowland RRR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Research, 2010, 154(1/2): 1-6. |
| [2] | Zhou L, Yang HC. Porcine reproductive and respiratory syndrome in China. Virus Research, 2010, 154(1/2): 31-37. |
| [3] | Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine, 2009, 27(28): 3704-3718. |
| [4] | Snijder EJ, Meulenberg JJ. The molecular biology of arteriviruses. The Journal of General Virology, 1998, 79(Pt5): 961-979. |
| [5] | Duan X, Nauwynck HJ, Pensaert MB. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV). Archives of Virology, 1997, 142(12): 2483-2497. |
| [6] | Lee YJ, Park CK, Nam E, Kim SH, Lee OS, Lee DS, Lee C. Generation of a porcine alveolar macrophage cell line for the growth of porcine reproductive and respiratory syndrome virus. Journal of Virological Methods, 2010, 163(2): 410-415. |
| [7] | Fire A. RNA-triggered gene silencing. Trends in Genetics, 1999, 15(9): 358-363. |
| [8] | Zhou JH, Rossi JJ. Progress in RNAi-based antiviral therapeutics. Methods in Molecular Biology, 2011, 721: 67-75. |
| [9] | Boden D, Pusch O, Silbermann R, Lee F, Tucker L, Ramratnam B. Enhanced gene silencing of HIV-1 specific siRNA using microRNA designed hairpins. Nucleic Acids Research, 2004, 32(3): 1154-1158. |
| [10] | Liu YP, Haasnoot J, Ter Brake O, Berkhout B, Konstantinova P. Inhibition of HIV-1 by multiple siRNAs expressed from a single microRNA polycistron. Nucleic Acids Research, 2008, 36(9): 2811-2824. |
| [11] | Xia B, Song HQ, Chen Y, Zhang XY, Xia XL, Sun HC. Efficient inhibition of porcine reproductive and respiratory syndrome virus replication by artificial microRNAs targeting the untranslated regions. Archives of Virology, 2013, 158(1): 55-61. |
| [12] | Zhu L, Bao LP, Zhang XY, Xia XL, Sun HC. Inhibition of porcine reproductive and respiratory syndrome virus replication with exosome-transferred artificial microRNA targeting the 3' untranslated region. Journal of Virological Methods, 2015, 223: 61-68. |
| [13] | Zhu L, Song HQ, Zhang XY, Xia XL, Sun HC. Inhibition of porcine reproductive and respiratory syndrome virus infection by recombinant adenovirus-and/or exosome-delivered the artificial microRNAs targeting sialoadhesin and CD163 receptors. Virology Journal, 2014, 11(1): 225. |
| [14] | Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Gorcyca D, Chladek D. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. Journal of Veterinary Diagnostic Investigation, 1992, 4(2): 117-126. |
| [15] | Hu SP, Zhang Z, Cai XH, Tian ZJ, Liu YG, An TQ, Wu DL. Valuation of protective efficacy of PRRS attenuated vaccine and inactivated mutant PRRSV strains. Chinese Journal of Preventive Veterinary Medicine, 2009, 31(5): 392-396. (in Chinese)胡守萍, 张卓, 蔡雪辉, 田志军, 刘永刚, 安同庆, 吴东来. 猪繁殖与呼吸综合征病毒弱毒疫苗和变异株灭活苗的免疫效果评价. 中国预防兽医学报, 2009, 31(5): 392-396. |
| [16] | Tian KG, Yu XL, Zhao TZ, Feng YJ, Cao Z, Wang CB, Hu Y, Chen XZ, Hu DM, Tian XS, Liu D, Zhang S, Deng XY, Ding YQ, Yang L, Zhang YX, Xiao HX, Qiao MM, Wang B, Hou LL, Wang XY, Yang XY, Kang LP, Sun M, Jin P, Wang SJ, Kitamura Y, Yan JH, Gao GF. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One, 2007, 2(6): e526. |
| [17] | Jacobs AC, Hermann JR, Mu?oz-Zanzi C, Prickett JR, Roof MB, Yoon KJ, Zimmerman JJ. Stability of porcine reproductive and respiratory syndrome virus at ambient temperatures. Journal of Veterinary Diagnostic Investigation, 2010, 22(2): 257-260. |
| [18] | Khare R, Chen CY, Weaver EA, Barry MA. Advances and future challenges in adenoviral vector pharmacology and targeting. Current Gene Therapy, 2011, 11(4): 241-258. |
| [19] | Zsengellér Z, Otake K, Hossain SA, Berclaz PY, Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. Journal of Virology, 2000, 74(20): 9655-9667. |
