 , 杨天志1, 连加攀1, 孟令左2, 王娜3, 刘维涛1
, 杨天志1, 连加攀1, 孟令左2, 王娜3, 刘维涛1

1. 南开大学环境科学与工程学院污染过程与基准教育部重点实验室, 天津市城市生态环境修复与污染防治重点实验室, 天津 300350;
2. 沈阳建筑大学市政与环境工程学院, 沈阳 110168;
3. 天津晟泽环保设备有限公司, 天津 300382
收稿日期: 2020-04-20; 修回日期: 2020-05-28; 录用日期: 2020-05-28
基金项目: 国家自然科学基金项目(No.41471411);天津市应用基础与前沿研究计划顶目(No.15JCYBJC22700)
作者简介: 吴佳妮(1996-), 女, E-mail:2316965518@qq.com
通讯作者(责任作者): 刘维涛(1979—), 男, 副教授/硕士生导师, 主要从事污染生态修复与生态毒理学研究.E-mail:lwt@nankai.edu.cn
摘要:为探讨微塑料对农作物的生态毒理效应,研究了两种粒径(20 nm和100 nm)聚苯乙烯纳米塑料(Polystyrene Nanoplastics,PSNPs)在6个浓度梯度(0、50、100、200、500和1000 mg·L-1)下对大豆(Glycine max)种子发芽和幼苗生长的影响.结果表明,两种粒径PSNPs均抑制种子发芽能力(发芽势、发芽指数、活力指数、平均发芽速度和发芽率),其中,发芽抑制率在一定程度上与暴露时间呈负相关关系.20 nm和100 nm PSNPs对根长、芽长和苗长的影响整体呈"低浓度促进,中高浓度抑制"的规律.20 nm PSNPs对根尖数量无显著影响,而100 nm PSNPs则表现出促进作用.20 nm和100 nm PSNPs对根直径和干重均有抑制作用.综上,PSNPs的植物毒性与粒径和浓度密切相关,在中等浓度(200 mg·L-1)时,其对大豆幼苗生长的毒害作用最大.
关键词:微塑料/纳米塑料聚苯乙烯大豆(Glycine max)种子发芽幼苗生长
Effects of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of soybean (Glycine max)
WU Jiani1
 , YANG Tianzhi1, LIAN Jiapan1, MENG Lingzuo2, WANG Na3, LIU Weitao1
, YANG Tianzhi1, LIAN Jiapan1, MENG Lingzuo2, WANG Na3, LIU Weitao1

1. Key Laboratory of Pollution Processes and Environmental Criteria, Ministry of Education, Tianjin Key Laboratory of Urban Ecology Environmental Remediation and Pollution Control, College of Environmental Science and Engineering, Nankai University, Tianjin 300350;
2. College of Municipal and Environment Engineering, Shenyang Jianzhu University, Shenyang 110168;
3. Tianjin Shengze Environmental Protection Equipment Co., Ltd., Tianjin 300382
Received 20 April 2020; received in revised from 28 May 2020; accepted 28 May 2020
Abstract: The influences of polystyrene nanoplastics (PSNPs) with two particle sizes (20 nm and 100 nm) on soybean (Glycine max) seed germination and seedling growth were studied to explore the ecotoxicological effects of microplastics on crops. PSNPs concentrations was designed with 0,50,100,200,500 and 1000 mg·L-1. The results showed that PSNPs (20 nm and 100 nm) inhibited the seed germination capacity (germination energy,germination index,vigor index,mean germination speed and germination rate) of Glycine max. Moreover,the germination inhibition rate was negatively correlated to the exposure time to a certain extent. PSNPs (20 nm and 100 nm) promoted the root length,bud length and seedling length of Glycine max treated with low concentration,but suppressed these parameters at medium to high concentration. 20 nm PSNPs had no significant effect on the number of root tips of Glycine max,while 100 nm PSNPs showed a positive effect. PSNPs (20 nm and 100 nm) had a negative impact on the root diameter and dry weight of Glycine max. Overall,the phytotoxicity of PSNPs is closely related to particle size and level. 200 mg·L-1 (intermediate level) PSNPs had the most toxic effect on seedling growth of Glycine max.
Keywords: microplastics/nanoplasticspolystyrenesoybean (Glycine max)seed germinationseedling growth
1 引言(Introduction)随着塑料产量的剧增, 塑料污染问题也已引起学术界和公众的广泛关注.据欧洲塑料协会统计, 2018年全球塑料产量高达3.59亿t, 中国塑料产量约占全球的30%(Plastics Europe, 2019). 2010年, 因管理不当而产生的塑料垃圾仅前20名国家就已达到2646万t(Jambeck et al., 2015).进入环境中的塑料经风化和光照等作用能够形成塑料碎片(Plastic Debris), 并引发重大的环境污染问题(Heddagaard et al., 2020).
微塑料(Microplastics)的概念最早由Thompson等(2004)提出, 目前通常认为微塑料是指粒径小于5 mm的塑料颗粒, 其中, 粒径为1~100 nm的塑料颗粒又被称为纳米塑料(Nanoplastics)(Hartmann et al., 2019).微塑料被认为是一种全球性新型污染物, 普遍存在于大气(Gasperi et al., 2018)、水体(Schmidt et al., 2017)和土壤(Wang et al., 2019)等环境介质中, 甚至极地地区也有微塑料被检出的报道(Peeken et al., 2018).微塑料粒径小且降解周期长, 易被生物体摄取并随之在食物链中迁移, 尤其是微塑料可作为多氯联苯(PCBs) (Mohamed Nor et al., 2019)、抗生素(Li et al., 2018)、农药(Wang et al., 2020)和重金属(Godoy et al., 2019)等污染物的载体, 从而对生态环境安全和人体健康形成潜在风险(Horton et al., 2017).鉴于上述原因, 2014年联合国环境规划署(UNEP)将微塑料列为全球十大环境问题之一(Qi et al., 2019).
目前, 有关微塑料/纳米塑料的研究主要为环境调查类研究, 集中于海洋(Thompson et al., 2004; Zhao et al., 2018; Liu et al., 2020)、海岸(Abayomi et al., 2017; Ruiz-Orejón et al., 2018)、河口(Xu et al., 2018; Han et al., 2020)、河流(Han et al., 2020; Zhang et al., 2020)和湖泊(Wang et al., 2018; Dong et al., 2020)等水域生态系统, 已开展的微塑料/纳米塑料生态毒理学研究集中于水生生物如藻类(Wu et al., 2019; Yi et al., 2019)、浮游动物(Botterell et al., 2019; Saavedra et al., 2019)、鱼类(Mak et al., 2019; Roch et al., 2019)和贝类(Digka et al., 2018; Magara et al., 2019; Rist et al., 2019)等方面.与水生生态系统相比, 陆地生态系统特别是农业生态系统中微塑料/纳米塑料污染的研究相对较少(Piehl et al., 2018), 已有研究证实农业土壤中的微塑料会对土壤结构、微生物活性、蚯蚓健康和植物生长等产生不良影响(Rodriguez-Seijo et al., 2017; de Souza Machado et al., 2019).据估算, 随着塑料薄膜和含有微塑料农业化学品的大量使用, 农业土壤中的微塑料积累量比海洋环境更多(de Souza Machado et al., 2018).高等植物尤其是粮食作物对保持农业生态系统的平衡起至关重要的作用, 但目前有关微塑料/纳米塑料的植物生态毒理学效应研究仍处于起步阶段(Bosker et al., 2019).
截至目前, 仅有少数文献开展了微塑料/纳米塑料对植物如绿豆(Vigna radiata)(刘蓥蓥等, 2019)、小麦(Triticum aestivum L.)(连加攀等, 2019; Lian et al., 2020)、洋葱(Allium cepa)(Giorgetti et al., 2020)和水芹(Lepidium sativum)(Bosker et al., 2019)等的影响研究.其中, 刘蓥蓥等(2019)发现23~38 μm聚乙烯微塑料在最高暴露浓度100 mg·g-1时对绿豆(Vigna radiata)幼苗的生长和水分吸收表现出显著抑制作用; 连加攀等(2019)发现乙烯-乙酸乙烯酯共聚物、线性低密度聚乙烯和聚甲基丙烯酸甲酯3种微塑料在0~1000 mg·L-1内对小麦(Triticum aestivum L.)的根长、芽长、苗长和干重基本均无显著影响; Lian等(2020)则表明100 nm聚苯乙烯纳米塑料在低暴露浓度0.1 mg·L-1时能显著促进小麦(Triticum aestivum L.)的生物量和叶绿素含量(p<0.01);另外, 据Giorgetti等(2020)报道, 50 nm聚苯乙烯纳米塑料能够内化于洋葱(Allium cepa)根分生区细胞, 引起氧化胁迫, 产生细胞毒性(如有丝分裂异常)和基因毒性.显然, 不同类型微塑料/纳米塑料对不同类型植物幼苗生长产生的影响有所不同.这与微塑料/纳米塑料的自身特性、植物响应和环境介质密切相关.因此, 若要全面和深入揭示微塑料/纳米塑料的植物毒性, 必然需要开展不同微塑料/纳米塑料类型及粒径, 以及不同植物品种和不同暴露环境的毒理学研究.而有关聚苯乙烯纳米塑料(Polystyrene nanoplastics, PSNPs)对于大豆的毒理学效应, 目前尚不清楚也未见报道.
种子萌发是植物生命周期的一个关键时期, 也是对外界环境因子最敏感的时期之一.因此, 开展种子发芽试验有望初步评估污染物对植物的毒理学效应(连加攀等, 2019).聚苯乙烯(Polystyrene, PS)是最常用的塑料聚合物之一, 也是目前研究微塑料生物积累和毒理学效应的模式微塑料(Xie et al., 2020).大豆作为重要的粮食作物, 占全球油籽年产量的40%, 具有重要的经济价值(Cao et al., 2017).此外, 豆科植物根瘤内拥有共生固氮的根瘤菌(Müller et al., 2016), 与之前已开展微塑料毒理学研究的植物差异较大.因此, 本研究以大豆作为供试植物, 以PSNPs作为研究对象.由于植物细胞壁孔的直径范围为5~30 nm(Tan et al., 2018), 本研究选取两种粒径(20 nm和100 nm)的PSNPs, 研究不同粒径和浓度PSNPs对大豆种子发芽和幼苗生长的影响, 旨在探讨微塑料对大豆的毒性效应, 为后期评估微塑料对植物的毒性效应及作用机制提供基础数据和科学依据.
2 材料与方法(Materials and methods)2.1 供试材料20 nm和100 nm固含量5%的PSNPs标准尺寸微球均购于上海辉质生物科技有限公司, 微球均一性均小于3%, 其分散介质均为去离子水和痕量表面活性剂(SDS).大豆种子购于天津市津南区种子公司.
2.2 纳米塑料表征采用场发射透射电子显微镜(Tecnai G2 F20, FEI, USA)观察PSNPs的形貌特征, 并使用Image J软件统计计算PSNPs微粒的平均粒径.
PSNPs悬浮液制备参考Giorgetti等(2020)的浓度设置.分别取2 mL 20 nm和100 nm PSNPs原液稀释5倍, 得到1×104 mg·L-1不同粒径的PSNPs悬浮液.吸取250、500、1000、2500和5000 μL 1×104 mg·L-1 PSNPs悬浮液, 用超纯水定容于50 mL容量瓶, 20 ℃左右水浴超声10 min (600 W, 40 kHz), 得到浓度依次为50、100、200、500和1000 mg·L-1不同粒径的PSNPs悬浮液.同时, 按上述条件做空白对照.使用纳米粒度与Zeta电位及分子量分析仪(Nano Series ZS90, Malvern, Britain)测定20 nm和100 nm不同浓度PSNPs悬浮液的粒径分布和Zeta电位.
2.3 种子发芽试验参照Lin等(2007)的试验操作方法, 本试验共11个处理组, 每个处理组3个平行.选取颗粒均匀饱满的大豆种子, 3% H2O2消毒30 min后, 用蒸馏水彻底冲洗3次, 以除去种子表面残留的H2O2, 再将其置于蒸馏水中浸泡12 h.将配制好的PSNPs悬浮液分别加入垫有滤纸的9 cm玻璃培养皿中, 每皿10 mL.随后将浸泡好的种子整齐摆放于滤纸上, 每皿10粒.
培养皿置于恒温培养箱的黑暗环境中培养7 d(约22 ℃), 于每晚8:00记录大豆种子的萌芽情况(发芽以种子胚根长度达到种子长度的1/2为标准).按式(1)~(5)分别计算种子的发芽势(Germination Energy, RE)、发芽指数(Germination Index, GI)、活力指数(Vigor Index, VI)、平均发芽速度(Mean Germination Speed, MGS)和发芽率(Germination Rate, GR).
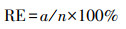 | (1) |
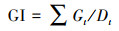 | (2) |
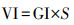 | (3) |
 | (4) |
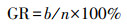 | (5) |
采用根际扫描仪(WinRHIZO Pro 2005b, Regent instruments Inc., Canada)测定大豆幼苗的根际参数.最后, 将大豆幼苗置于103 ℃烘箱中烘干至恒重, 测得其干重.
2.4 数据处理实验结果均表示为平均值±标准偏差.实验数据均使用SPSS 16.0进行单因素方差分析, 以LSD或Dunnett′s T3检验进行事后多重比较, 除非另有其他说明.
3 结果与讨论(Results and discussion)3.1 纳米塑料表征PSNPs标准尺寸微球的形貌特征如图 1所示.通过透射电镜观察发现, PSNPs微球较为均匀地分散于体系中, 且颗粒呈较规则的球形(图 1a和1b).从粒径分布图来看, PSNPs微球的粒径均呈正态分布, 20 nm PSNPs微球的平均粒径为19.34 nm, 100 nm PSNPs微球的平均粒径为99.58 nm (图 1c和1d).
图 1(Fig. 1)
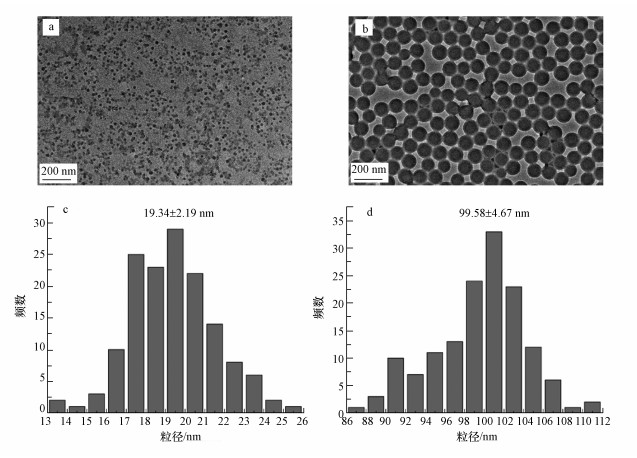 |
| 图 1 20 nm (a, c)和100 nm(b, d) PSNPs微球的透射电镜图和粒径分布图 Fig. 1Transmission electron microscopy and particle size distribution of 20 nm (a, c) and 100 nm (b, d) PSNPs microspheres |
20 nm PSNPs在各浓度下的水合粒径均大于24 nm, 且悬浮液浓度为50 mg·L-1时, 其水合粒径达到31.52 nm; 而100 nm PSNPs在各浓度下的水合粒径均接近微球的原始粒径(图 2).这表明相较于100 nm PSNPs, 20 nm PSNPs在悬浮液中更易出现团聚现象. Zeta电位的绝对值能够反映颗粒分散体的稳定性(Tan et al., 2018). 20 nm和100 nm PSNPs悬浮液的Zeta电位值均在-40~-70 mV之间(图 2).这表明各浓度下两种粒径PSNPs的悬浮液稳定性良好.
图 2(Fig. 2)
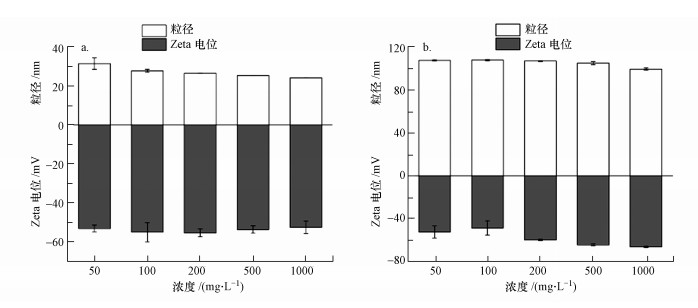 |
| 图 2 20 nm (a)和100 nm (b) PSNPs不同浓度悬浮液的粒径和Zeta电位 Fig. 2Particle size and Zeta potential of 20 nm (a) and 100 nm (b) PSNPs suspensions with different concentrations |
3.2 PSNPs对种子活力的影响以发芽势、发芽指数、活力指数和平均发芽速度等为指标表征PSNPs对大豆种子活力的影响.结果显示, 20 nm和100 nm不同浓度PSNPs均抑制大豆种子的发芽势、发芽指数、活力指数和平均发芽速度(表 1).整体而言, 在中等浓度(200 mg·L-1)时, 20 nm PSNPs对种子活力等指标的抑制程度达到最大, 与对照差异显著(p<0.05);在低浓度(50 mg·L-1)或高浓度(1000 mg·L-1)时, 100 nm PSNPs对种子活力等指标的抑制程度达到最大, 与对照差异显著(p<0.05), 而20 nm PSNPs对大豆种子发芽势、发芽指数和平均发芽速度均无显著影响.这与PSNPs的粒径相关.李连祯等(2019)证实200 nm PS可被生菜(Lactuca sativa)根部吸收富集, 从地下向地上部转运, 积累分布于茎叶, 但未发现1 μm PS被生菜根部所吸收. Larue等(2012)进行的类似研究表明, TiO2纳米颗粒在小麦(Triticum aestivum L.)中的吸收具有尺寸依赖性, 小于36 nm的颗粒能从根部转移到叶片, 40~140 nm之间的颗粒保留在根部, 大于140 nm的颗粒则不被植物所吸收.因此, 与工程纳米材料类似, 微塑料的粒径是影响其环境行为的一个重要因子(Tan et al., 2018; Bosker et al., 2019).粒径超过植物细胞壁孔尺寸上限的微塑料或纳米材料能够进入植物体内, 一方面, 可能是由于微塑料或纳米材料能够作用于植物使其细胞壁孔增大(Tan et al., 2018);另一方面, 可能是由于植物细胞对微塑料或纳米材料具有内吞作用(Triebskorn et al., 2019).此外, 微塑料的形状和表面电荷等特性在其转运与归趋的过程中也发挥作用(Eerkes-Medrano et al., 2018; Lambert et al., 2018).细胞膜由阴离子亲水外表面组成, 因此, 阳离子颗粒比中性或阴离子颗粒更易附着于细胞表面(Zhu et al., 2013).这在Feng等(2020)的研究中表现为:带正电荷的氨基改性聚苯乙烯纳米塑料(PS-NH2)对铜绿微囊藻(Microcystis aeruginosa)细胞密度的抑制作用强于带负电荷的磺酸改性聚苯乙烯纳米塑料(PS-SO3H).
表 1(Table 1)
| 表 1 PSNPs对大豆种子活力的影响 Table 1 Effects of PSNPs on the vigor of Glycine max seeds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 1 PSNPs对大豆种子活力的影响 Table 1 Effects of PSNPs on the vigor of Glycine max seeds
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.3 PSNPs对种子发芽率的影响从总体上来看, 20 nm和100 nm不同浓度PSNPs对大豆种子的发芽率均表现出抑制作用, 但存在一定程度的时间-效应负相关关系(图 3), 即随着暴露时间的增加, 暴露于两种粒径PSNPs的大豆发芽抑制率趋于减弱.第1 d, 暴露于20 nm PSNPs的大豆发芽抑制率高达10.00%~70.00%, 暴露于100 nm PSNPs的大豆发芽抑制率高达55.00%~85.00%.第7 d, 大豆发芽抑制率则分别降至3.57%~25.00%和8.93%~19.64%(图 3).微塑料能够吸附在种子、胚根和根毛的表面(Bosker et al., 2019), 且植物在种子萌芽阶段对水分需求极高.因此, 产生上述现象的原因可能在于吸附在种皮孔囊上的塑料会降低植物对水分的吸收速度(Calero et al., 1981), 进而在试验初期抑制或延迟植物的种子发芽, 当暴露时间增加, 植物自身吸取充足的水分, 微塑料对种子萌芽的负效应则逐渐消失.在Bosker等(2019)对水芹(Lepidium sativum)的研究中, 发现3种不同粒径(50、500和4800 nm)的微塑料在暴露8 h时均显著抑制其种子发芽率, 且具有尺寸和剂量相关性, 但在暴露24 h后, 3种粒径的微塑料对水芹的发芽率则没有影响. Giorgetti等(2020)也发现PSNPs暴露72 h后对洋葱(Allium cepa)的种子发芽无影响.本研究结果与之相似.
图 3(Fig. 3)
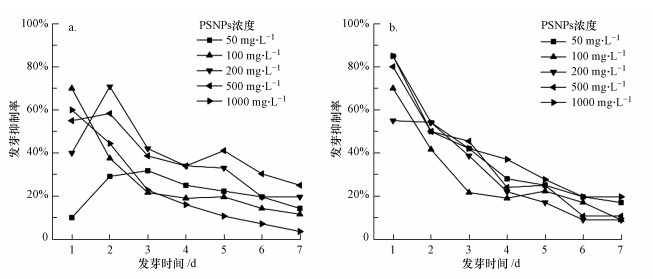 |
| 图 3 20 nm (a)和100 nm (b) PSNPs对大豆种子发芽率的影响 Fig. 3Effects of 20 nm (a) and 100 nm (b) PSNPs on the germination rate of Glycine max seeds |
在中等浓度(200 mg·L-1)时, 20 nm PSNPs对大豆种子发芽率的抑制作用强于100 nm PSNPs; 而在低浓度(50 mg·L-1)和高浓度(1000 mg·L-1)时, 100 nm PSNPs对大豆种子发芽率的抑制作用较20 nm PSNPs更强.两种不同粒径的PSNPs在不同浓度下抑制程度的不同是由微塑料本身的“化合物生物毒性效应”、“物理毒性效应”和“环境介质”等因素的细微变化所共同决定的.目前, 尚不清楚其具体作用机制(Liu et al., 2020).
综上, 不论是总体还是局部的变化趋势与规律, PSNPs对大豆种子发芽率的影响与其对大豆种子活力的影响基本一致.这表明两者的影响是相关联的, 即PSNPs通过影响大豆的种子活力进而影响大豆种子的发芽率, 但随着暴露时间的增加, 其对后者的影响逐渐减小.
3.4 PSNPs对幼苗生长的影响大豆幼苗的生长情况如图 4所示. 20 nm PSNPs对大豆幼苗的干重、根尖数量无显著影响, 而100 nm PSNPs在500 mg·L-1 (p<0.01)和1000 mg·L-1 (p<0.05)时显著抑制大豆幼苗的干重, 在100 mg·L-1 (p<0.01)、500 mg·L-1 (p<0.01)和1000 mg·L-1 (p<0.001)时显著促进大豆幼苗的根尖数. 20 nm和100 nm不同浓度PSNPs对大豆幼苗的根直径均表现出抑制作用, 当浓度为50 mg·L-1时, 与对照组相比, 分别显著抑制30.30%和40.91%(图 5a~5c).20 nm和100 nm不同浓度PSNPs对大豆幼苗根长、芽长和苗长的总体影响在一定程度上呈“低浓度促进, 中高浓度抑制”的规律, 同时, 部分处理组在高浓度时出现“抑制作用减弱”的现象(图 5d~5f).这可能在于, 低浓度时微塑料更易被植物所吸收, 进而引起植物体内代谢谱的改变(Lian et al., 2020).鉴于微塑料与工程纳米材料的相似性, 在Hong等(2005)的研究中发现纳米TiO2能提高叶绿体类囊体膜上Mg2+-ATPase的活性, 且本课题组的前期研究表明, PSNPs能够促进小麦(Triticum aestivum L.)幼苗生长时的光合作用(Lian et al., 2020).因此推测, 微塑料或许能够作用于植物的光系统进而促进植物生长.而高浓度时, 随着暴露时间的增加, 微塑料极易出现团聚现象(Alimi et al., 2018), 形成尺寸较大的团聚体, 阻碍植物对其的吸收, 同时, 比表面积的降低也减少了种子与微塑料之间的接触面积, 这或许是PSNPs对大豆幼苗生长的抑制作用得到缓解的原因之一.结合以上原因, 联系微塑料的粒径、浓度和表面电荷等自身特性及植物对微塑料的响应等多种因素, 微塑料在中等浓度时可能对植物造成严重的物理堵塞或其它损伤, 使得抑制作用达到最大.在本研究中具体表现为:当浓度为中等浓度(200 mg·L-1)时, 20 nm PSNPs对大豆幼苗生长的抑制程度最强(图 6).这与图 4中所展现的内容是相符的.
图 4(Fig. 4)
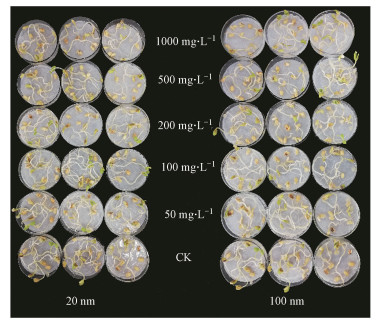 |
| 图 4 大豆的幼苗生长情况 Fig. 4Growth of Glycine max |
图 5(Fig. 5)
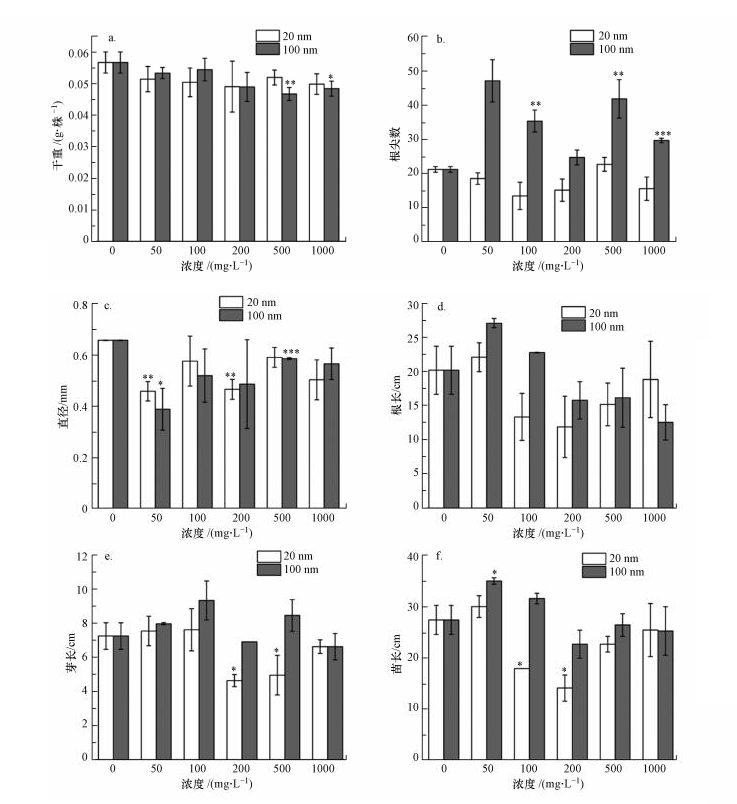 |
| 图 5 PSNPs对大豆干重(a)、根尖数(b)、根直径(c)、根长(d)、芽长(e)和苗长(f)的影响 (同一粒径下, 对照组与其他不同浓度处理组的显著性差异分别用“*”(p<0.05, t检验)、“**”(p < 0.01, t检验)和“***”(p<0.001, t检验)表示) Fig. 5Effects of PSNPs on the dry weight (a), root tips (b), diameter (c), root length (d), bud length (e) and seedling length (f) of Glycine max |
图 6(Fig. 6)
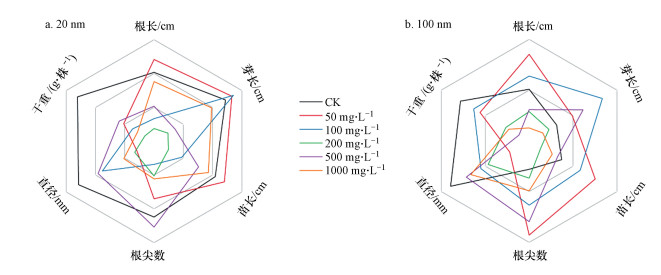 |
| 图 6 20 nm (a)和100 nm (b) PSNPs对大豆幼苗生长影响的总览图 Fig. 6Overview of the effects of 20 nm (a) and 100 nm (b) PSNPs on the growth of Glycine max seedlings |
4 结论(Conclusions)1) 20m和100 nm PSNPs主要通过吸附于大豆种皮表面, 从而降低种子吸收水分的速度, 进而抑制种子活力.
2) 20 nm和100 nm PSNPs均可抑制大豆种子的发芽率, 且发芽抑制率与暴露时间之间呈一定的负相关关系. PSNPs通过对大豆种子活力的影响进而影响种子的发芽率, 因而两组实验结果的变化趋势具有相似性.
3) 整体而言, 20 nm和100 nm PSNPs对大豆幼苗生长的抑制作用在中等浓度(200 mg·L-1)时达到最大.这可能在于:此浓度时, PSNPs团聚体的直径恰好达到某个临界值, 对大豆根系造成严重的物理损害; 而当浓度增大, PSNPs团聚体的直径超过此临界值后, 与植物根系的接触面积越来越小且不易于进入植物体内, 抑制程度得到缓解.
参考文献
| Abayomi O A, Range P, Al-Ghouti M A, et al. 2017. Microplastics in coastal environments of the Arabian Gulf[J]. Marine Pollution Bulletin, 124(1): 181-188. DOI:10.1016/j.marpolbul.2017.07.011 |
| Alimi O S, Farner Budarz J, Hernandez L M, et al. 2018. Microplastics and nanoplastics in aquatic environments:Aggregation, deposition, and enhanced contaminant transport[J]. Environmental Science & Technology, 52(4): 1704-1724. |
| Bosker T, Bouwman L J, Brun N R, et al. 2019. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum[J]. Chemosphere, 226: 774-781. DOI:10.1016/j.chemosphere.2019.03.163 |
| Botterell Z L R, Beaumont N, Dorrington T, et al. 2019. Bioavailability and effects of microplastics on marine zooplankton:A review[J]. Environmental Pollution, 245: 98-110. DOI:10.1016/j.envpol.2018.10.065 |
| Calero E, West S H, Hinson K. 1981. Water absorption of soybean seeds ans associated causal factors[J]. Crop Science, 21(6): 926-933. DOI:10.2135/cropsci1981.0011183X002100060030x |
| Cao Z, Stowers C, Rossi L, et al. 2017. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.)[J]. Environmental Science:Nano, 4(5): 1086-1094. DOI:10.1039/C7EN00015D |
| De Souza Machado A A, Kloas W, Zarfl C, et al. 2018. Microplastics as an emerging threat to terrestrial ecosystems[J]. Global Change Biology, 24(4): 1405-1416. DOI:10.1111/gcb.14020 |
| De Souza Machado A A, Lau C W, Kloas W, et al. 2019. Microplastics can change soil properties and affect plant performance[J]. Environmental Science & Technology, 53(10): 6044-6052. |
| Digka N, Tsangaris C, Torre M, et al. 2018. Microplastics in mussels and fish from the Northern Ionian Sea[J]. Marine Pollution Bulletin, 135: 30-40. DOI:10.1016/j.marpolbul.2018.06.063 |
| Dong M, Luo Z, Jiang Q, et al. 2020. The rapid increases in microplastics in urban lake sediments[J]. Scientific Reports, 10(1): 848. DOI:10.1038/s41598-020-57933-8 |
| Eerkes-Medrano D, Thompson R. 2018. Microplastic Contamination in Aquatic Environments[M]. . |
| Feng L J, Sun X D, Zhu F P, et al. 2020. Nanoplastics promote microcystin synthesis and release from cyanobacterial Microcystis aeruginosa[J]. Environmental Science & Technology, 54(6): 3386-3394. |
| Gasperi J, Wright S L, Dris R, et al. 2018. Microplastics in air:Are we breathing it in?[J]. Current Opinion in Environmental Science & Health, 1: 1-5. |
| Giorgetti L, Spanò C, Muccifora S, et al. 2020. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination:Internalization in root cells, induction of toxicity and oxidative stress[J]. Plant Physiology and Biochemistry, 149: 170-177. DOI:10.1016/j.plaphy.2020.02.014 |
| Godoy V, Blázquez G, Calero M, et al. 2019. The potential of microplastics as carriers of metals[J]. Environmental Pollution, 255: 113363. DOI:10.1016/j.envpol.2019.113363 |
| Han M, Niu X, Tang M, et al. 2020. Distribution of microplastics in surface water of the lower Yellow River near estuary[J]. Science of the Total Environment, 707: 135601. DOI:10.1016/j.scitotenv.2019.135601 |
| Hartmann N B, Hüffer T, Thompson R C, et al. 2019. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris[J]. Environmental Science & Technology, 53(3): 1039-1047. |
| Heddagaard F E, M?ller P. 2020. Hazard assessment of small-size plastic particles:Is the conceptual framework of particle toxicology useful?[J]. Food and Chemical Toxicology, 136: 111106. DOI:10.1016/j.fct.2019.111106 |
| Hong F, Zhou J, Liu C, et al. 2005. Effect of Nano-TiO2 on photochemical reaction of chloroplasts of spinach[J]. Biological Trace Element Research, 105(1/3): 269-279. |
| Horton A A, Walton A, Spurgeon D J, et al. 2017. Microplastics in freshwater and terrestrial environments:Evaluating the current understanding to identify the knowledge gaps and future research priorities[J]. Science of the Total Environment, 586: 127-141. DOI:10.1016/j.scitotenv.2017.01.190 |
| Jambeck J R, Geyer R, Wilcox C, et al. 2015. Plastic waste inputs from land into the ocean[J]. Science, 347(6223): 768-771. DOI:10.1126/science.1260352 |
| Lambert S, Wagner M. 2018. Freshwater Microplastics:Emerging Environmental Contaminants?[M]. . |
| Larue C, Laurette J, Herlin-Boime N, et al. 2012. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.):Influence of diameter and crystal phase[J]. Science of the Total Environment, 431: 197-208. DOI:10.1016/j.scitotenv.2012.04.073 |
| Li J, Zhang K, Zhang H. 2018. Adsorption of antibiotics on microplastics[J]. Environmental Pollution, 237: 460-467. DOI:10.1016/j.envpol.2018.02.050 |
| 李连祯, 周倩, 尹娜, 等. 2019. 食用蔬菜能吸收和积累微塑料[J]. 科学通报, 64(9): 928-934. |
| 连加攀, 沈玫玫, 刘维涛. 2019. 微塑料对小麦种子发芽及幼苗生长的影响[J]. 农业环境科学学报, 38(4): 737-745. |
| Lian J, Wu J, Xiong H, et al.2020.Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.)[J].Journal of Hazardous Materials, 385: 121620 |
| Lin D, Xing B. 2007. Phytotoxicity of nanoparticles:Inhibition of seed germination and root growth[J]. Environmental Pollution, 150(2): 243-250. DOI:10.1016/j.envpol.2007.01.016 |
| Liu G, Jiang R, You J, et al. 2020. Microplastic impacts on microalgae growth:effects of size and humic acid[J]. Environmental Science & Technology, 54(3): 1782-1789. |
| Liu T, Zhao Y, Zhu M, et al. 2020. Seasonal variation of micro-and meso-plastics in the seawater of Jiaozhou Bay, the Yellow Sea[J]. Marine Pollution Bulletin, 152: 110922. DOI:10.1016/j.marpolbul.2020.110922 |
| 刘蓥蓥, 张旗, 崔文智, 等. 2019. 聚乙烯微塑料对绿豆发芽的毒性研究[J]. 环境与发展, 31(5): 123-125. |
| Magara G, Khan F R, Pinti M, et al. 2019. Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis)[J]. Journal of Toxicology and Environmental Health, Part A, 82(10): 616-625. DOI:10.1080/15287394.2019.1633451 |
| Mak C W, Ching-Fong Yeung K, Chan K M. 2019. Acute toxic effects of polyethylene microplastic on adult zebrafish[J]. Ecotoxicology and Environmental Safety, 182: 109442. DOI:10.1016/j.ecoenv.2019.109442 |
| Mohamed Nor N H, Koelmans A A. 2019. Transfer of PCBs from microplastics under simulated gut fluid conditions is biphasic and reversible[J]. Environmental Science & Technology, 53(4): 1874-1883. |
| Müller D B, Vogel C, Bai Y, et al. 2016. The plant microbiota:Systems-level insights and perspectives[J]. Annual Review of Genetics, 50(1): 211-234. DOI:10.1146/annurev-genet-120215-034952 |
| Peeken I, Primpke S, Beyer B, et al. 2018. Arctic sea ice is an important temporal sink and means of transport for microplastic[J]. Nature Communications, 9(1): 1505. DOI:10.1038/s41467-018-03825-5 |
| Piehl S, Leibner A, L?der M G J, et al. 2018. Identification and quantification of macro-and microplastics on an agricultural farmland[J]. Scientific Reports, 8(1): 17950. DOI:10.1038/s41598-018-36172-y |
| Plastics Europe.2019.Plastics-the Facts 2019 An analysis of European plastics production, demand and waste data[R].Brussels: Association of Plastic Manufacturers |
| Qi R, Jones D L, Li Z, et al. 2019. Behavior of microplastics and plastic film residues in the soil environment:A critical review[J]. Science of the Total Environment, 703: 134722. |
| Rist S, Baun A, Almeda R, et al. 2019. Ingestion and effects of micro-and nanoplastics in blue mussel (Mytilus edulis) larvae[J]. Marine Pollution Bulletin, 140: 423-430. DOI:10.1016/j.marpolbul.2019.01.069 |
| Roch S, Walter T, Ittner L D, et al. 2019. A systematic study of the microplastic burden in freshwater fishes of south-western Germany-Are we searching at the right scale?[J]. Science of the Total Environment, 689: 1001-1011. DOI:10.1016/j.scitotenv.2019.06.404 |
| Rodriguez-Seijo A, Louren?o J, Rocha-Santos T A P, et al. 2017. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché[J]. Environmental Pollution, 220: 495-503. DOI:10.1016/j.envpol.2016.09.092 |
| Ruiz-Orejón L F, Sardá R, Ramis-Pujol J. 2018. Now, you see me:High concentrations of floating plastic debris in the coastal waters of the Balearic Islands (Spain)[J]. Marine Pollution Bulletin, 133: 636-646. |
| Saavedra J, Stoll S, Slaveykova V I. 2019. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton[J]. Environmental Pollution, 252: 715-722. DOI:10.1016/j.envpol.2019.05.135 |
| Schmidt C, Krauth T, Wagner S. 2017. Export of plastic debris by rivers into the sea[J]. Environmental Science & Technology, 51(21): 12246-12253. |
| Tan W, Peralta-Videa J R, Gardea-Torresdey J L. 2018. Interaction of titanium dioxide nanoparticles with soil components and plants:current knowledge and future research needs:a critical review[J]. Environmental Science:Nano, 5(2): 257-278. DOI:10.1039/C7EN00985B |
| Thompson R C, Olsen Y, Mitchell R P, et al. 2004. Lost at sea:Where is all the plastic?[J]. Science, 304(5672): 838-838. DOI:10.1126/science.1094559 |
| Triebskorn R, Braunbeck T, Grummt T, et al. 2019. Relevance of nano-and microplastics for freshwater ecosystems:A critical review[J]. TrAC Trends in Analytical Chemistry, 110: 375-392. DOI:10.1016/j.trac.2018.11.023 |
| Wang J, Liu X, Li Y, et al. 2019. Microplastics as contaminants in the soil environment:A mini-review[J]. Science of the Total Environment, 691: 848-857. DOI:10.1016/j.scitotenv.2019.07.209 |
| Wang T, Yu C, Chu Q, et al. 2020. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films[J]. Chemosphere, 244: 125491. DOI:10.1016/j.chemosphere.2019.125491 |
| Wang W, Yuan W, Chen Y, et al. 2018. Microplastics in surface waters of Dongting Lake and Hong Lake, China[J]. Science of the Total Environment, 633: 539-545. DOI:10.1016/j.scitotenv.2018.03.211 |
| Wu Y, Guo P, Zhang X, et al. 2019. Effect of microplastics exposure on the photosynthesis system of freshwater algae[J]. Journal of Hazardous Materials, 374: 219-227. DOI:10.1016/j.jhazmat.2019.04.039 |
| Xie X, Deng T, Duan J, et al. 2020. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway[J]. Ecotoxicology and Environmental Safety, 190: 110133. DOI:10.1016/j.ecoenv.2019.110133 |
| Xu P, Peng G, Su L, et al. 2018. Microplastic risk assessment in surface waters:A case study in the Changjiang Estuary, China[J]. Marine Pollution Bulletin, 133: 647-654. DOI:10.1016/j.marpolbul.2018.06.020 |
| Yi X, Chi T, Li Z, et al. 2019. Combined effect of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa[J]. Environmental Science and Pollution Research, 26(15): 15011-15018. DOI:10.1007/s11356-019-04865-0 |
| Zhang L, Liu J, Xie Y, et al. 2020. Distribution of microplastics in surface water and sediments of Qin river in Beibu Gulf, China[J]. Science of the Total Environment, 708: 135176. DOI:10.1016/j.scitotenv.2019.135176 |
| Zhao J, Ran W, Teng J, et al. 2018. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China[J]. Science of The Total Environment, 640-641: 637-645. DOI:10.1016/j.scitotenv.2018.05.346 |
| Zhu M, Nie G, Meng H, et al. 2013. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate[J]. Accounts of Chemical Research, 46(3): 622-63. DOI:10.1021/ar300031y |
